Aspartase mutant, recombinant expression vector containing aspartase mutant, recombinant bacterium and application thereof
A technology of aspartase and expression vector, which is applied in the field of genetic engineering, can solve the problems of harsh requirements, low enzyme activity, and high production cost of β-amino acids, and achieve the effects of strengthening capacity, improving enzyme activity, and improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Contains the construction of the recombinant expression vector of the gene encoding aspartase mutant
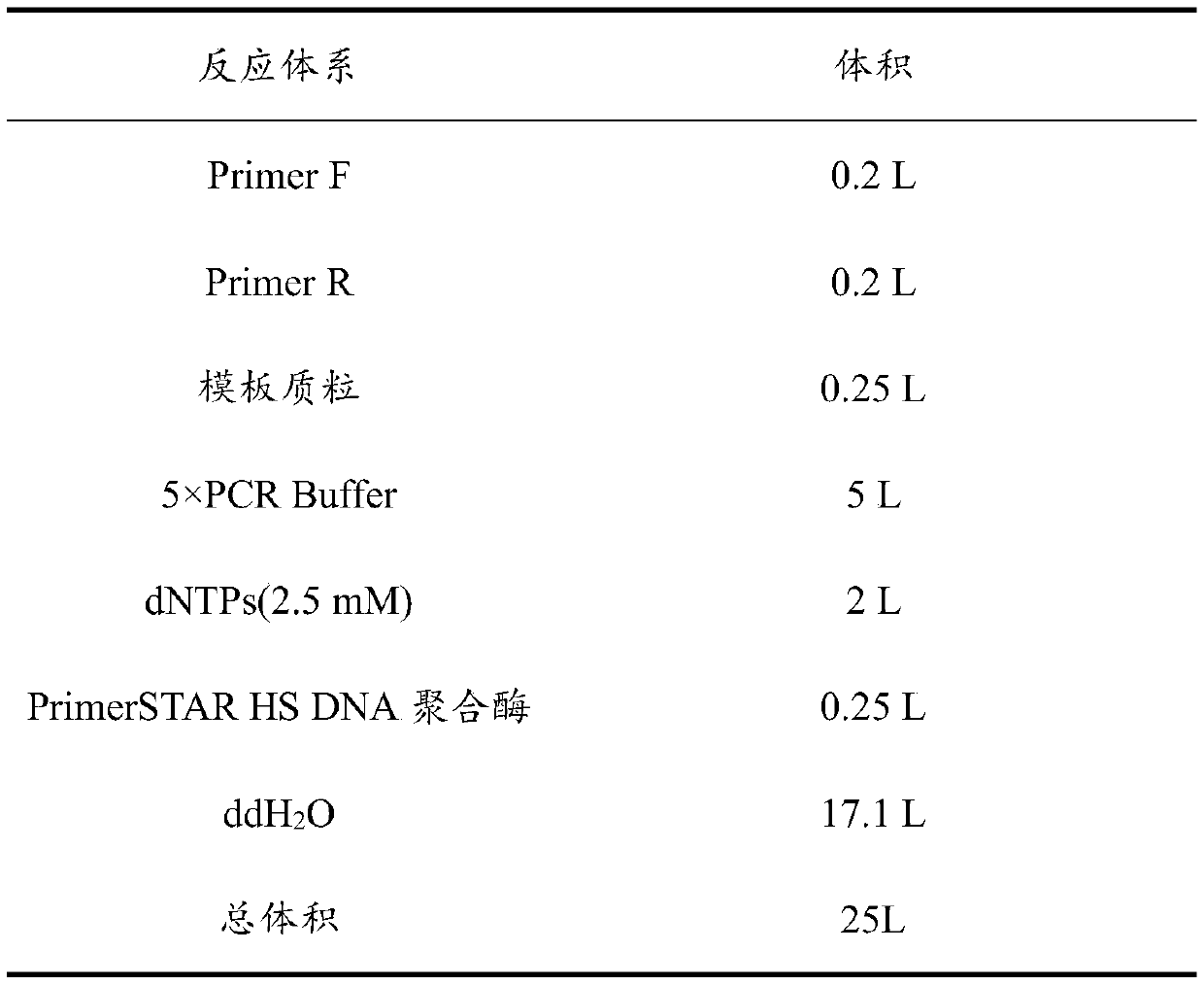
[0032] With the pET-21a recombinant plasmid containing the nucleotide sequence shown in SEQ ID NO: 4 as a template, Fprimer (sequence shown in SEQ ID NO: 5) and Rprimer (sequence shown in SEQ ID NO: 6) as primers, The mutant plasmid was constructed by the two-step PCR method of the whole plasmid (the reaction system is shown in Table 1, and the reaction conditions are shown in Table 2), and the gene E427Q shown in SEQ ID NO: 3 was obtained.
[0033] Table 1 PCR reaction system
[0034]
[0035] Table 2 PCR reaction conditions
[0036]
[0037]
[0038] The PCR product was checked by gel electrophoresis, and then 1 μL of Dpn I restriction endonuclease was added to 20 μL of the PCR product to digest the template plasmid, and incubated overnight at 25° C. or 37° C. for 3 to 4 hours. Pipette 5 μL of the digested product and transform it into Escheri...
Embodiment 2
[0039] Embodiment 2 produces the recombinant escherichia coli engineering bacterium construction of aspartase mutant
[0040] The strain containing the correct recombinant plasmid pET21a-E427Q obtained in Example 1 is the recombinant strain pET21a-E427Q / E.coli BL21 of the present invention.
Embodiment 3
[0041] Example 3 Expression and Purification of Aspartase from Recombinant Bacteria pET21a-E427Q / E.coli BL21
[0042] The recombinant strain pET21a-E427Q / E.coli BL21 constructed in Example 2 and the control strain pET21a-AspB / E.coli BL21 expressing the unmutated original enzyme BsAspB (wild type, amino acid sequence shown in SEQ ID NO:3) Inoculate in 10mL ampicillin-containing LB medium respectively, culture overnight at 37°C with shaking, transfer to 50mL ampicillin-containing LB medium the next day at 1% inoculum size, culture at 37°C for 2-3 hours, then add 0.5mM IPTG was induced at 16°C for 12-16h. The cells were collected by centrifugation at 8000 rpm for 10 min at 4° C. and crushed, and the cell crushed supernatant (crude enzyme liquid) was collected for subsequent purification.
[0043] Purification of aspartase or aspartase mutants is carried out in a hot water bath at 60° C. for 30 minutes, and then centrifuged at 12000 rpm for 90 minutes to obtain the purified enzym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com