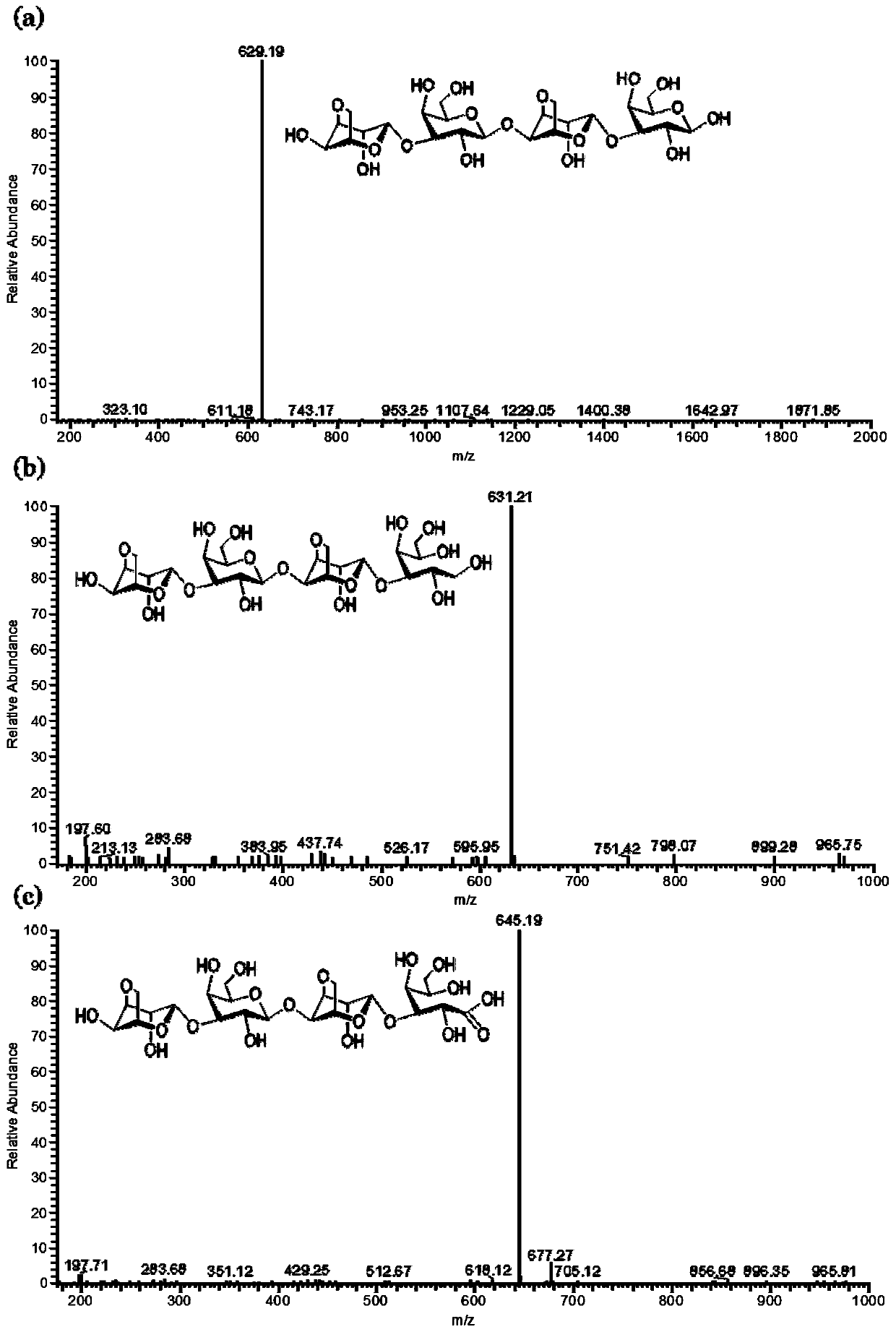
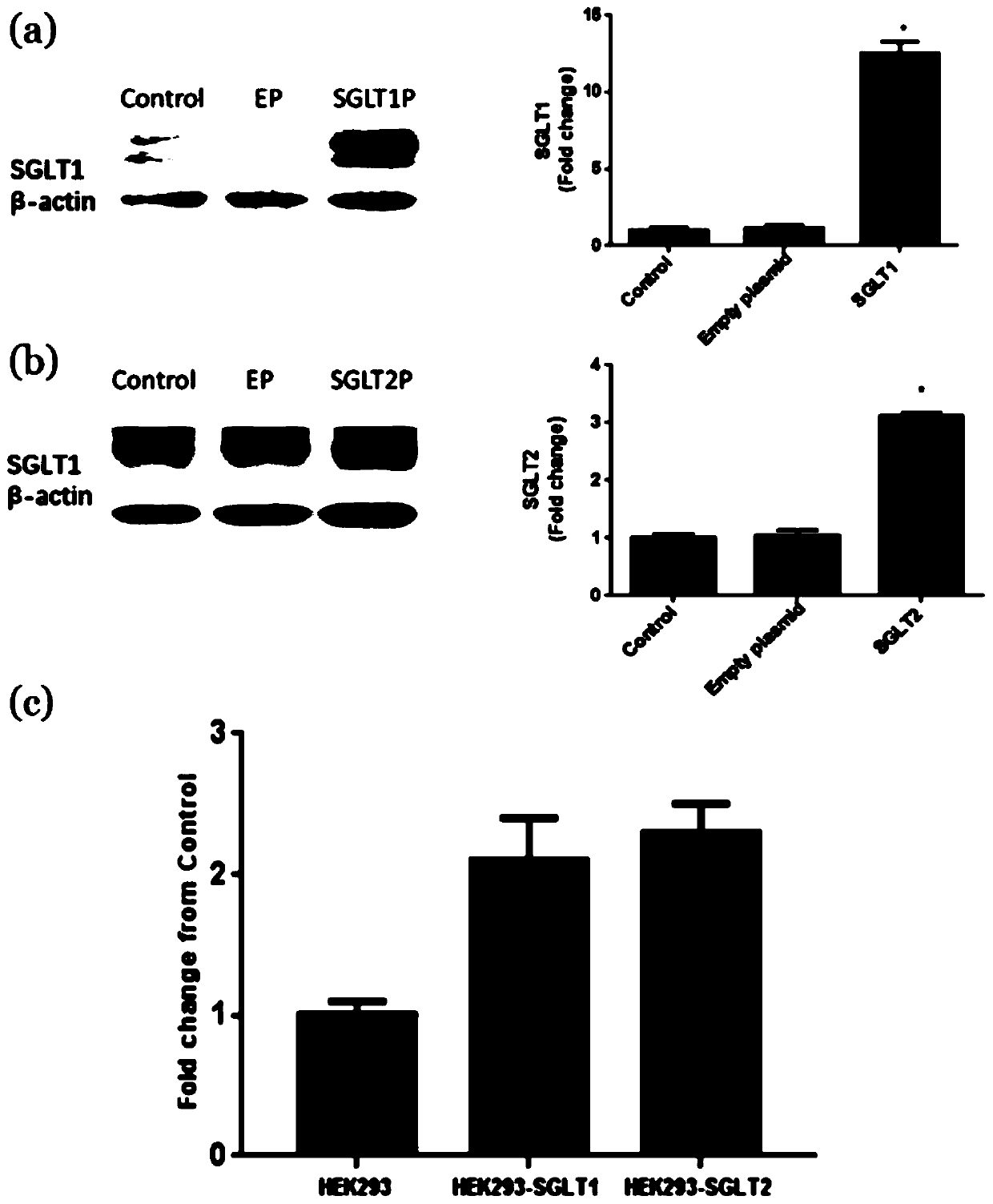
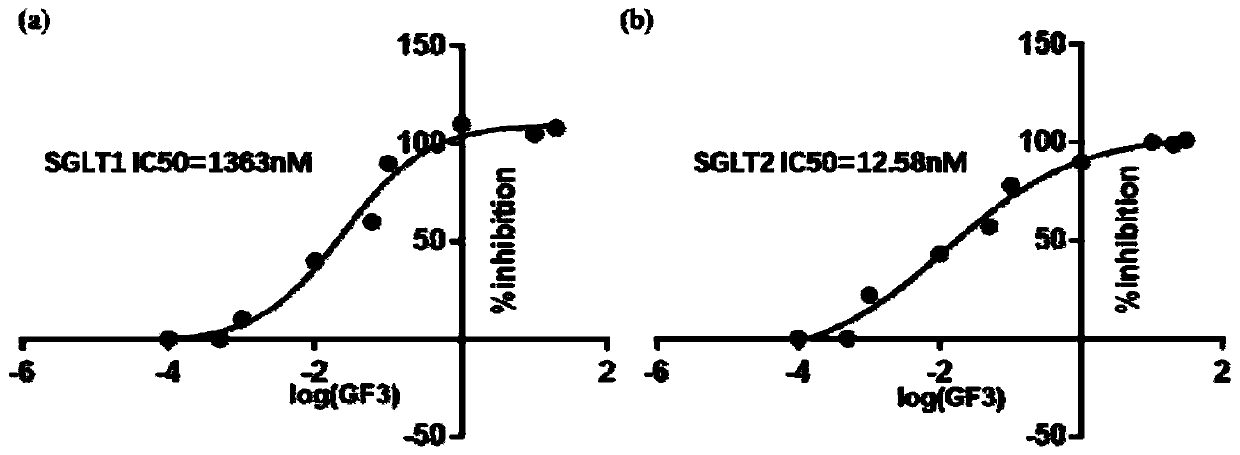
Application of galactooligosaccharides and derivatives of galactooligosaccharides as SGLT inhibitor
A galacto-oligosaccharide and derivative technology, applied in application, food science, drug combination and other directions, can solve problems such as safety hazards, and achieve the effect of improving fatty liver, lowering blood lipid and fatty liver, and improving insulin resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Containing 6-O-sulfate-β-1,3-D-galactose (Gal6S) and α-1,4-L-3,6-inner ether galactose (AnG) thiogaran oligosaccharide ( Preparation of SAOs), oligosaccharide alcohols (SAOs-OH) and oligosaccharide acids (SAOs-OOH).
[0040] Prepare 1000mg of sulfur agar polysaccharide with dilute sulfuric acid with a molar concentration of 0.1M to form a 10mg / mL aqueous solution, heat it to 60°C, stir and degrade it for 1.5 hours, neutralize it with an aqueous NaOH solution with a molar concentration of 2M after cooling, collect the supernatant by centrifugation, and then Add 3 volumes of 95% medical ethanol (concentration 95wt%) overnight at 4°C, centrifuge to collect the supernatant, remove the ethanol by rotary evaporation under reduced pressure, dialyze and desalt with a 200Da dialysis bag, concentrate by rotary evaporation, and freeze-dry to obtain SAOs. Take 100 mg of SAOs and dissolve it in 10 mL of 100 mM NaBH 4 The aqueous solution (containing NaOH with a molar con...
Embodiment 2
[0045] Example 2: Laver gum oligosaccharides (POs), oligosaccharide alcohols (POs) containing β-1,3-D-galactose (Gal) and 6-O-sulfate-α-1,4-galactose (Gal6S) -OH) and the preparation of oligosaccharide acids (POs-OOH).
[0046] Prepare laver gum with dilute sulfuric acid with a molar concentration of 0.1M to prepare a 10mg / mL aqueous solution, heat it to 80°C, stir and degrade it for 2.0 hours, neutralize it with an aqueous NaOH solution with a molar concentration of 2M after cooling, collect the supernatant by centrifugation, and add 4 times the volume 95% medical ethanol was placed overnight at 4°C, the supernatant was collected by centrifugation, and the ethanol was removed by rotary evaporation under reduced pressure, then desalted with a 200Da dialysis bag, concentrated by rotary evaporation, and freeze-dried to obtain laver gum POs. Take 150 mg of POs oligosaccharides and dissolve it in 15 ml of NaBH with a molar concentration of 150 mM 4 The aqueous solution (containin...
Embodiment 3
[0051] Example 3: Agar oligosaccharides containing β-1,3-D-galactose (Gal) and α-1,4-L-3,6-galactose (AnG) and their oligosaccharide alcohols and oligosaccharides acid preparation.
[0052] Dissolve the agarose in hot water, use dilute hydrochloric acid with a molar concentration of 0.1M to prepare a 10mg / mL solution, stir and degrade it at 80°C for 0.5 hours, neutralize it with an aqueous NaOH solution with a molar concentration of 2M after cooling, collect the supernatant by centrifugation, and then Add 2.5 times the volume of 95% medical ethanol at 4°C overnight, centrifuge to collect the supernatant, remove the ethanol by rotary evaporation, dialyze and desalt with a 200Da dialysis bag, concentrate by rotary evaporation and freeze-dry to obtain agar oligosaccharide AOs, and further reduce it with sodium borohydride to obtain agar Glucooligosaccharide alcohol AOs-OH, or agarooligosaccharide acid AOs-OOH obtained by oxidation with Benedict's reagent. The chemical structural...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com