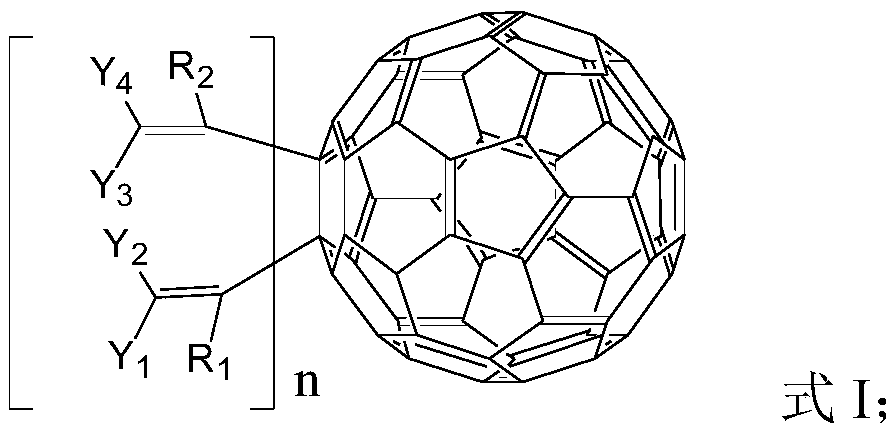
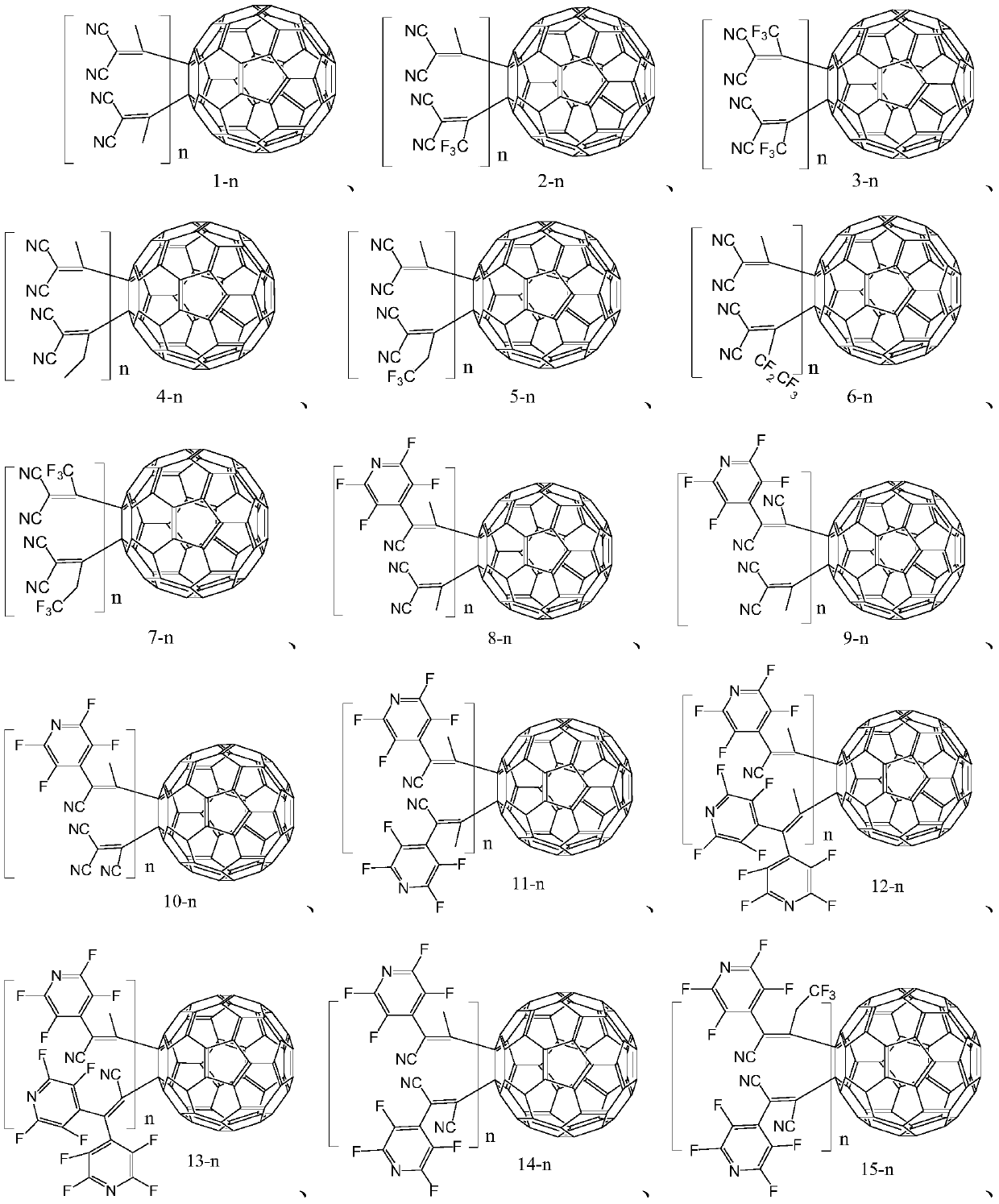
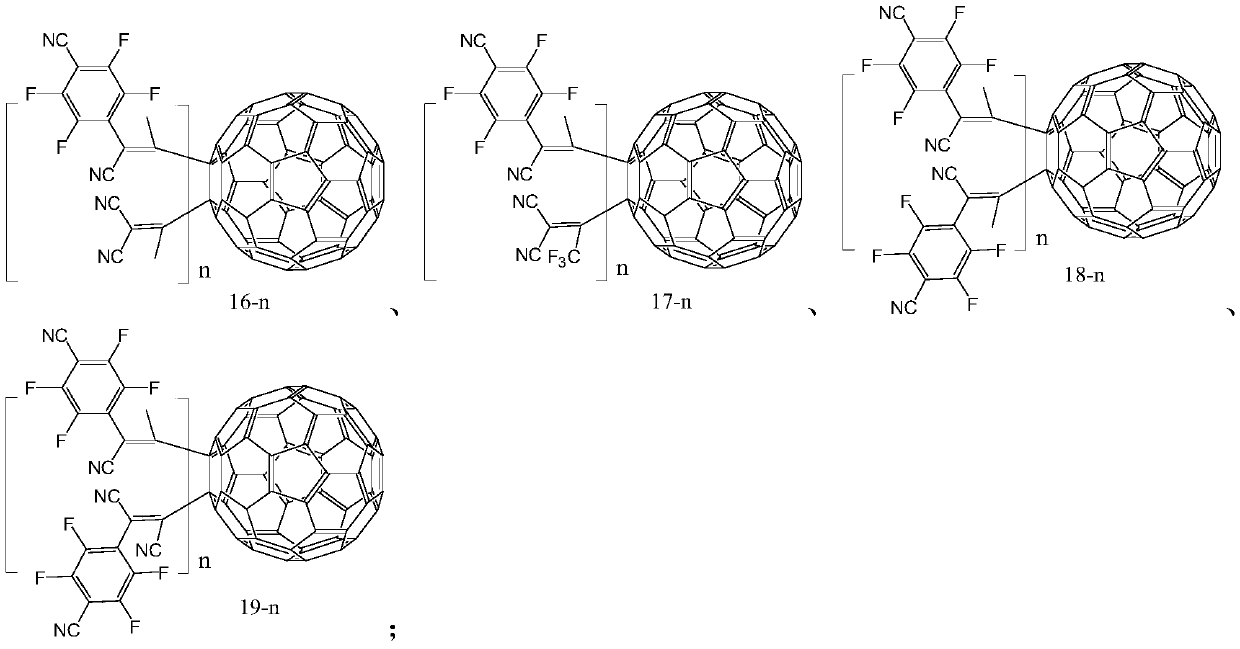
Fullerene derivative, application thereof and OLED device containing fullerene derivative
A technology of fullerene derivatives and devices, which is applied in the preparation of electric solid devices, semiconductor devices, organic compounds, etc. There are many problems such as the urgent need to develop hybrid materials to achieve the effects of high structural stability and thermal stability, improved binding rate, and good electron receiving ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0061] The preparation of compound 1-1, its synthetic route is as follows:
[0062]
[0063] Concrete synthetic steps are as follows:
[0064] (1) Anhydrous tetrahydrofuran (500 mL) was added to a 1000 mL three-necked flask, sodium hydride (28.8 g, 1.2 mol) was added under ice-bath conditions, and malononitrile (66.1 g, 1.0 mol) was slowly added dropwise under nitrogen protection,
[0065] After the dropwise addition, stir in an ice bath for 1 hour, then add acetyl chloride (78.5 g, 1.0 mol) dropwise, after the dropwise addition, stir at room temperature for 4 hours, slowly add methanol (10 mL), stir for 10 minutes, then add water ( ), extracted with ethyl acetate (300mL×2), combined the organic phases, washed once with water (200mL), washed once with saturated brine (100mL), dried over sodium sulfate, filtered, and spin-dried to obtain the crude product as a yellow solid (98.5g ), flash column purification (eluent: petroleum ether / ethyl acetate = 10:1-4:1, eluted in diffe...
preparation example 2
[0071] Compound 1-2 The preparation of the specific synthesis steps are as follows:
[0072] (1) Add compound B (13.8g, 0.050mol) into a 1000mL three-necked flask, protect it under nitrogen, inject re-distilled pyridine (150mL), stir and dissolve, add sodium methoxide (4.05g, 0.075mol), and then heat up to 80°C; After the reaction system was stirred at 80°C for 10 minutes, a solution of o-dichlorobenzene (500mL) in fullerene C60 (12.5g, 0.017mol) was added dropwise, and after the addition was completed, the reaction was continued at 80°C for 24 hours; After cooling to room temperature, the reaction solution was poured into methanol and left to stand for 4 hours before filtering, and the filter cake was redispersed and then subjected to column separation (toluene / petroleum ether=1:1) to obtain 6.7g of crude product; the resulting crude product was dissolved in Chloroform, after filtering through a 0.20 μm polytetrafluoroethylene (PTFE) filter membrane, a small amount of multi...
preparation example 3
[0076] The preparation of compound 16-1, its synthetic route is as follows:
[0077]
[0078] Concrete synthetic steps are as follows:
[0079] (1) Add anhydrous tetrahydrofuran (500mL) into a 1000mL three-necked flask, add sodium hydride (14.4g, 0.60mol) under ice bath conditions, and slowly add 4-cyanomethyl-2,3,5,6 -Tetrafluorobenzonitrile (compound C, 107.1g, 0.50mol), after the dropwise addition, stir in an ice bath for 1 hour, then add acetyl chloride (39.25g, 0.5mol) dropwise, after the dropwise addition, stir at room temperature for 4 hours, Slowly add methanol (10mL), stir for 10 minutes, add water (500mL), extract with ethyl acetate (300mL×2), combine the organic phases, wash once with water (200mL), wash once with saturated brine (100mL), sulfuric acid Sodium-dried, filtered, and spin-dried to obtain the crude product as a yellow solid (123.7 g), which was purified by flash column (eluent: petroleum ether / ethyl acetate=10:1-4:1, eluted in different proportions), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com