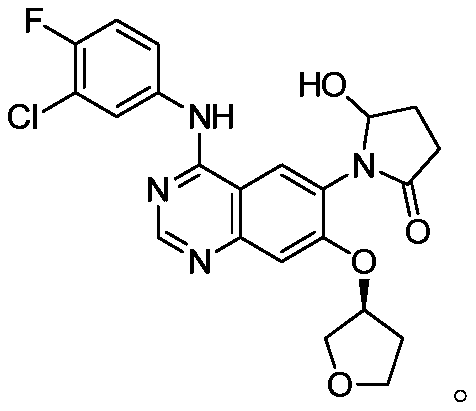
Afatinib dimaleate tablet and preparation method thereof
A technology of afatinib maleate and tinib tablets, which is applied in the field of afatinib maleate tablets and its preparation, can solve the problems of high substance content and low production efficiency, and improve the uniformity of mixing , simplified production process, and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The afatinib maleate tablet in the present embodiment is made up of tablet core and the film coating that is wrapped in outside the tablet core, and tablet core is made of the raw material of following quality (making 1000): maleic acid afatinib Tini (calculated as afatinib dimaleate) 44.34g, lactose (F100) 185.79g, microcrystalline cellulose (PH101) 27.72g, crospovidone 5.40g, silicon dioxide 1.35g, hard Magnesium fatty acid 5.40g; Film coating adopts the raw material of following quality (making 1000 tablets): film coating premix (Opadry) 10.80g, purified water 79.20g.
[0056] The preparation method of Afatinib maleate tablet, comprises the following steps:
[0057] Step (1) ingredients: afatinib maleate is granulated with a 40-mesh sieve, and set aside. Dry each auxiliary material at a temperature of 80±5°C, the moisture content of each auxiliary material is: lactose≤1.0%, microcrystalline cellulose≤2.0%, crospovidone≤4.0%, silicon dioxide≤4.0%, and set aside.
[...
Embodiment 2
[0063] The afatinib maleate tablet in the present embodiment is made up of tablet core and the film coating that is wrapped in outside the tablet core, and tablet core is made of the raw material of following quality (making 1000): maleic acid afatinib Tini (calculated as afatinib dimaleate) 44.34g, lactose (F100) 185.79g, microcrystalline cellulose (PH101) 33.72g, crospovidone 5.40g, silicon dioxide 1.35g, hard Magnesium fatty acid 5.40g; Film coating adopts the raw material of following quality (making 1000 tablets): film coating premix (Opadry) 11.04g, purified water 80.95g. The preparation method is the same as in Example 1.
Embodiment 3
[0065] The afatinib maleate tablet in the present embodiment is made up of tablet core and the film coating that is wrapped in outside the tablet core, and tablet core is made of the raw material of following quality (making 1000): maleic acid afatinib Tini (calculated as afatinib dimaleate) 44.34g, lactose (F100) 185.79g, microcrystalline cellulose (PH101) 27.72g, crospovidone 8.25g, silicon dioxide 1.35g, hard Magnesium fatty acid 5.40g; Film coating adopts the raw material of following quality (making 1000 tablets): film coating premix (Opadry) 10.91g, purified water 80.03g. The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com