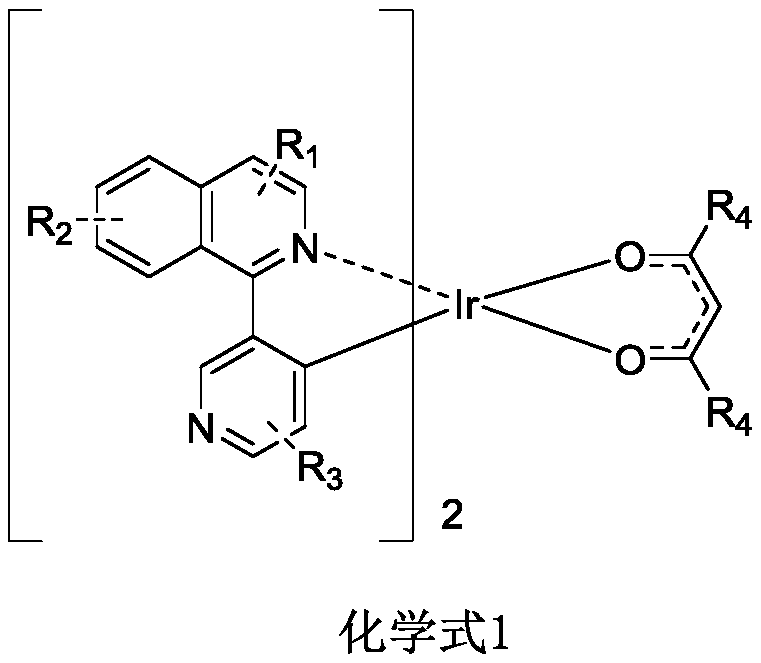
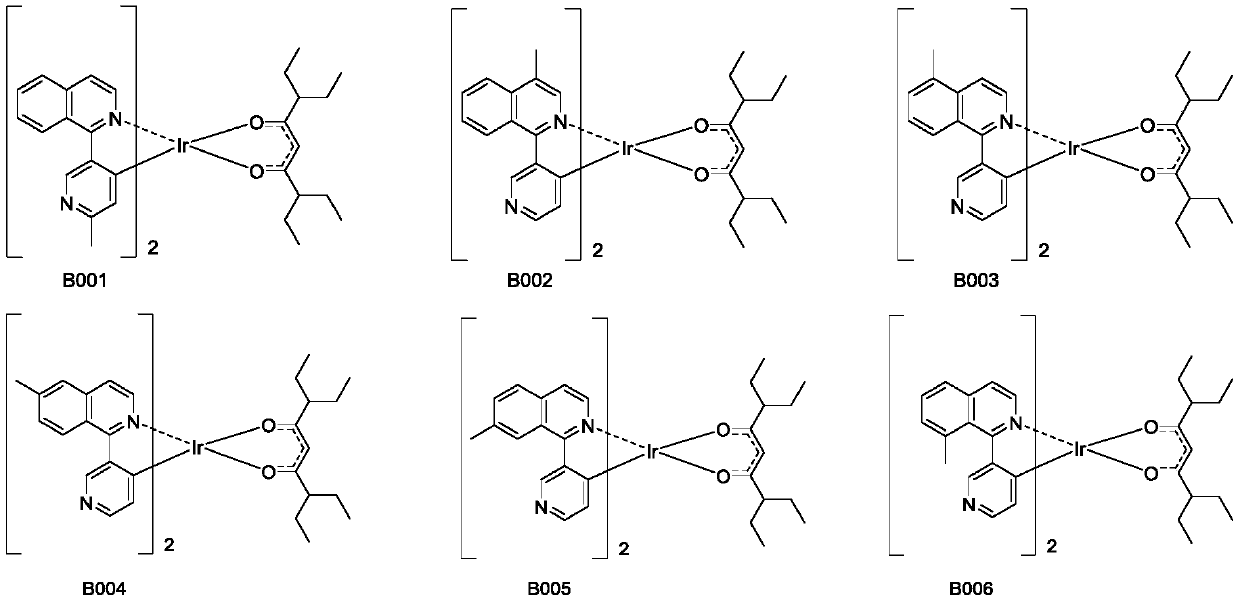
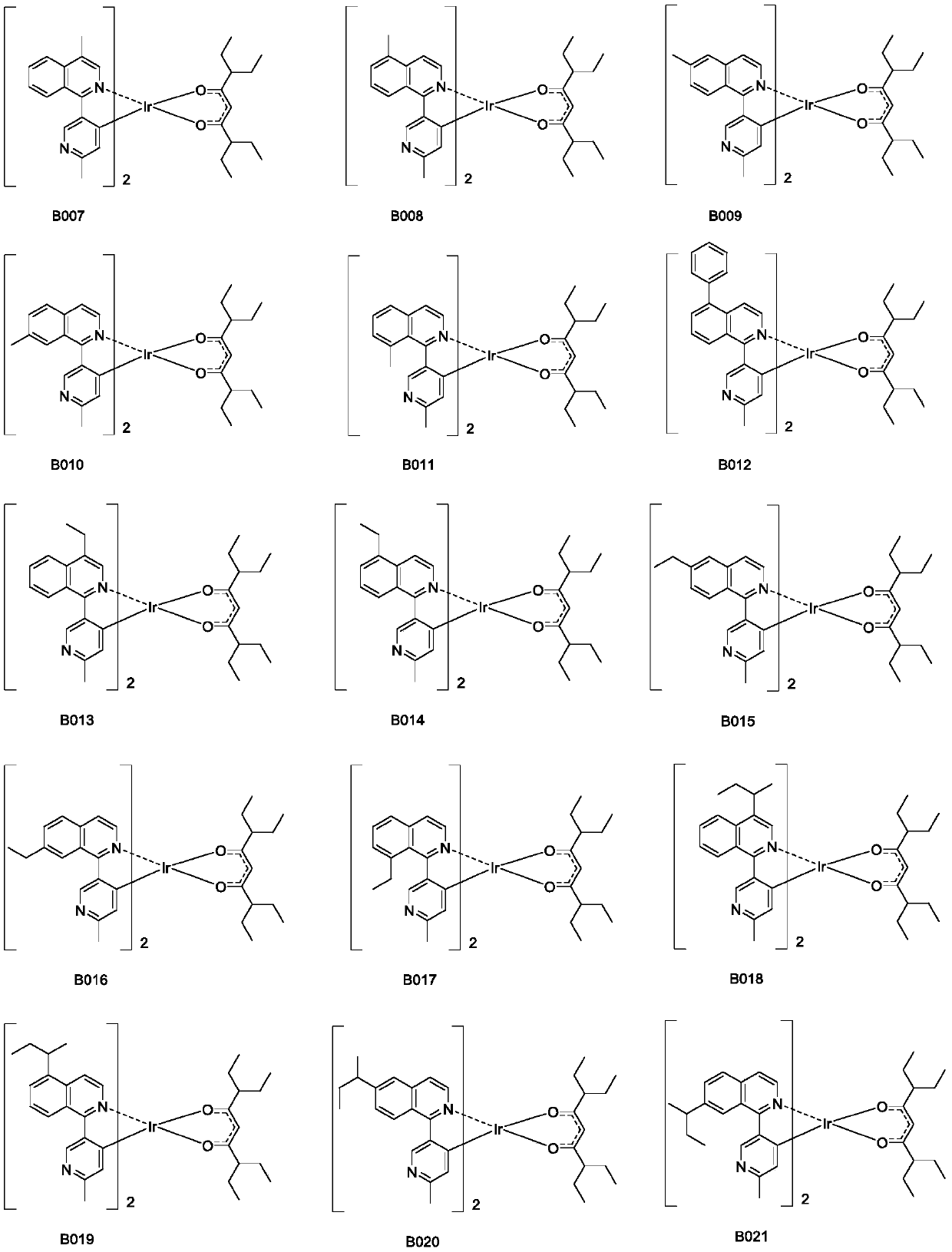
Organic iridium metal complex, preparation method thereof and organic electroluminescent devices
A technology of iridium metal complexes and complexes, applied in indium organic compounds, platinum group organic compounds, electric solid devices, etc., can solve the problems of late start, short life and poor thermal stability of red phosphorescent materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] An organic iridium metal complex B001, the specific synthesis steps are as follows:
[0053] 1) Under nitrogen protection system, weigh a-1 (90.8mmol, 20g), IrCl 3 ·3H 2 O (30.3mmol, 7.05g) was put into the reaction system, a mixed solution of 400mL ethylene glycol ether and 133mL purified water was added, and the reaction was refluxed under nitrogen protection (120°C) for 24 hours. After the reaction stopped, the system was cooled to room temperature, and there was precipitation. Precipitation, suction filtration of the precipitate, washing and drying with water, absolute ethanol and petroleum ether in sequence to obtain the bridging ligand b-1 (15.3 g, yield 75.8%) in the form of orange-red powder;
[0054] MW theoretical value: 1332.3, test value: 1330.2;
[0055] 2) Weigh orange-red powder bridging ligand b-1 (11.3mmol, 15g), add 7.2g of ligand 3,7-diethyl-4,6-dione c-1, and add to the system Add 400mL of ethylene glycol ether and 15.6g of potassium carbonate, un...
Embodiment 2
[0066] An organic iridium metal complex B109, the specific synthesis steps are as follows:
[0067] 1) Under nitrogen protection system, weigh a-109 (76.23mmol, 20g), IrCl 3 ·3H 2 O (25.4mmol, 8.9g) was put into the reaction system, a mixed solution of 400mL ethylene glycol ethyl ether and 133mL purified water was added, refluxed under nitrogen protection (120°C) for 24 hours, and then cooled to room temperature, a precipitate was precipitated, and the precipitate was Suction filtration, washing and drying with water, absolute ethanol and petroleum ether in sequence to obtain the bridging ligand b-109 (14.2 g, yield 74.5%) in the form of red powder;
[0068] MW theoretical value: 1500.7, test value: 1498.4;
[0069] 2) Weigh the red powder bridging ligand b-109 (9.3mmol, 14g), add 3.6g ligand heptane-3,5-dione c-109, then add 400mL ethylene glycol ether to the system and 12.9g of potassium carbonate, under nitrogen protection, stirred at 120°C for 24 hours, suction filtered...
Embodiment 3
[0080] An organic iridium metal complex B117, the specific synthesis steps are as follows:
[0081] 1) Under nitrogen protection system, weigh a-117 (96.9mmol, 20g), IrCl 3 ·3H 2 O (32.3mmol, 11.4g) was put into the reaction system, a mixed solution of 400mL ethylene glycol ethyl ether and 133mL pure water was added, refluxed under nitrogen protection (120°C) for 24 hours, and then cooled to room temperature, a precipitate was precipitated, and the precipitate was Suction filtration, washing and drying with water, absolute ethanol and petroleum ether in sequence to obtain bridged ligand b-117 (14.9 g, yield 72%) of orange-red powder;
[0082] MW theoretical value: 1276.2; test value: 1274.1;
[0083] 2) Weigh the bridging ligand b-117 (11.4mmol, 14.5g) of the orange-red powder, add 3.4g of the ligand pentane-2,4-dione c-117, and then add 400mL of ethylene glycol to the system Diethyl ether and 15.4g potassium carbonate were stirred at 120°C for 24 hours under the protection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com