CPV (canine parvovirus)-HuN1703 strain and application thereof
A technology of canine parvovirus, cpv-hun1703, applied in the direction of antiviral agents, virus antigen components, viruses/bacteriophages, etc., can solve gene variation, can not fully meet the needs of disease prevention and control and market consumption, inactivated vaccines and attenuated vaccines High protection level, high antibody production level, and good immunogenicity can be achieved due to problems such as decline in immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
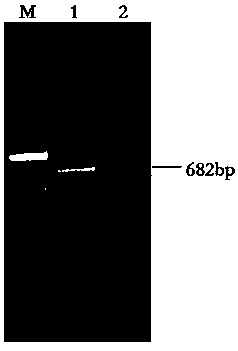
[0031] Example 1: Isolation and identification of canine parvovirus CPV-HuN1703 strain
[0032] 1.1 Source and collection of disease materials: collected from severely ill dogs admitted to a pet hospital in Hengyang City, Hunan Province, and disease materials after death.
[0033] It was clinically found that the dog was depressed, reluctant to eat, severe diarrhea with vomiting, red blood clots in the stool, and intestinal mucosal shedding. The existing medications can hardly prevent the deterioration of the condition. The stool was taken on-site and tested for CPV antigen with colloidal gold test strips. The test result was positive.
[0034] After the sick and dead dogs were disinfected, tissue disease materials such as the dog's heart, liver, spleen, lymph nodes, small intestine and intestinal contents were collected and stored at -70℃.
[0035] Other virus sources:
[0036] CPV standard strain (CVCC AV298), from China Veterinary Drug Administration (HA titer is 1:1024).
[0037] CP...
Embodiment 2
[0057] Example 2: Canine parvovirus CPV-HuN1703 strain is used for vaccine preparation
[0058] (1) Preparation of vaccine
[0059] An inactivated canine parvovirus vaccine prepared by the following method:
[0060] Step a, culture the cat kidney passage cells F81 according to the conventional method: the growth medium is MEM containing 5% fetal bovine serum (containing penicillin sodium and streptomycin sulfate 100 μg / mL each), at 37°C, 5% CO 2 Culture and subculture in an incubator. When the cells grow to 80% confluence, wash them with PBS once and then change to serum-free medium;
[0061] Step b, take the content as 5×10 4 TCID 50 / ml canine parvovirus CPV-HuN1703 strain was inoculated into the well-growing F81 cells prepared in step a at a 5% volume percentage, adsorbed at 37°C for 1 hour, discarded the adsorption solution, and added MEM containing 10 μg / mL trypsin Continue to cultivate as a maintenance fluid;
[0062] Step c: After inoculation, observe 3 times a day, record the c...
Embodiment 3
[0071] Example 3: Vaccine efficacy test
[0072] 3.1 Materials and methods
[0073] As a control, the CPV standard strain and the CPV-NY130615 strain were used according to the preparation method of the inactivated vaccine in Example 2 to prepare a batch of products. Chongbiwei® Youmiankang is selected as a commercial vaccine (mainly immune to canine fever, canine smallness, hepatitis and canine parainfluenza, batch number: A505A01)
[0074] Twenty-five 30-40-day-old hybrid puppies that were negative by pathogen detection and antibody detection were randomly divided into 5 groups with 5 in each group, named as control group, CPV-HuN1703 group, CPV standard group, CPV- NY130615 group and commercial vaccine group. The control group was injected with 50% ISA206 adjuvant (50% ISA206+50% saline), and the CPV-HuN1703 group, CPV standard group, and CPV-NY130615 group were injected with the 20180601 batch prepared in Example 2 The secondary vaccine is a vaccine prepared based on the CPV st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com