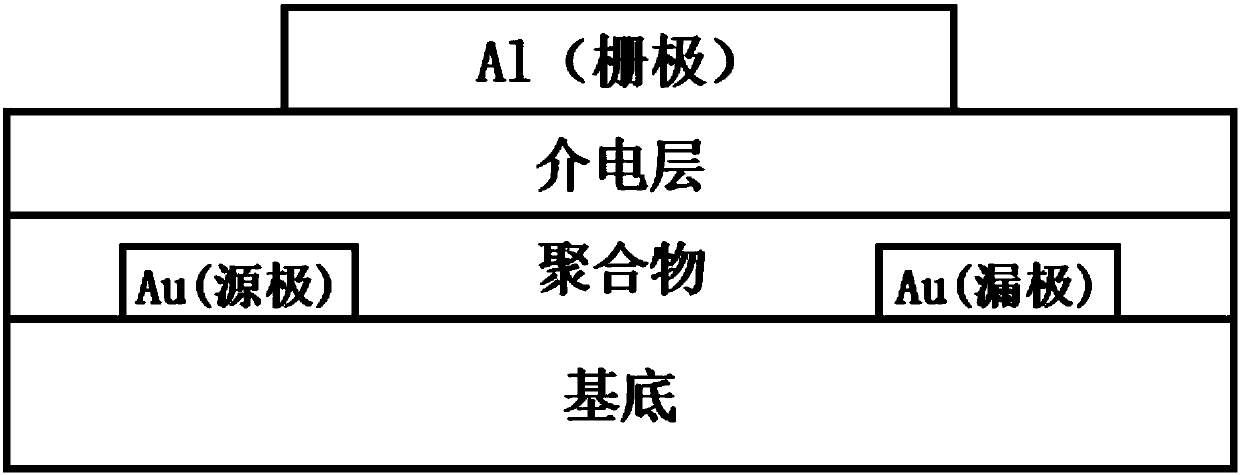
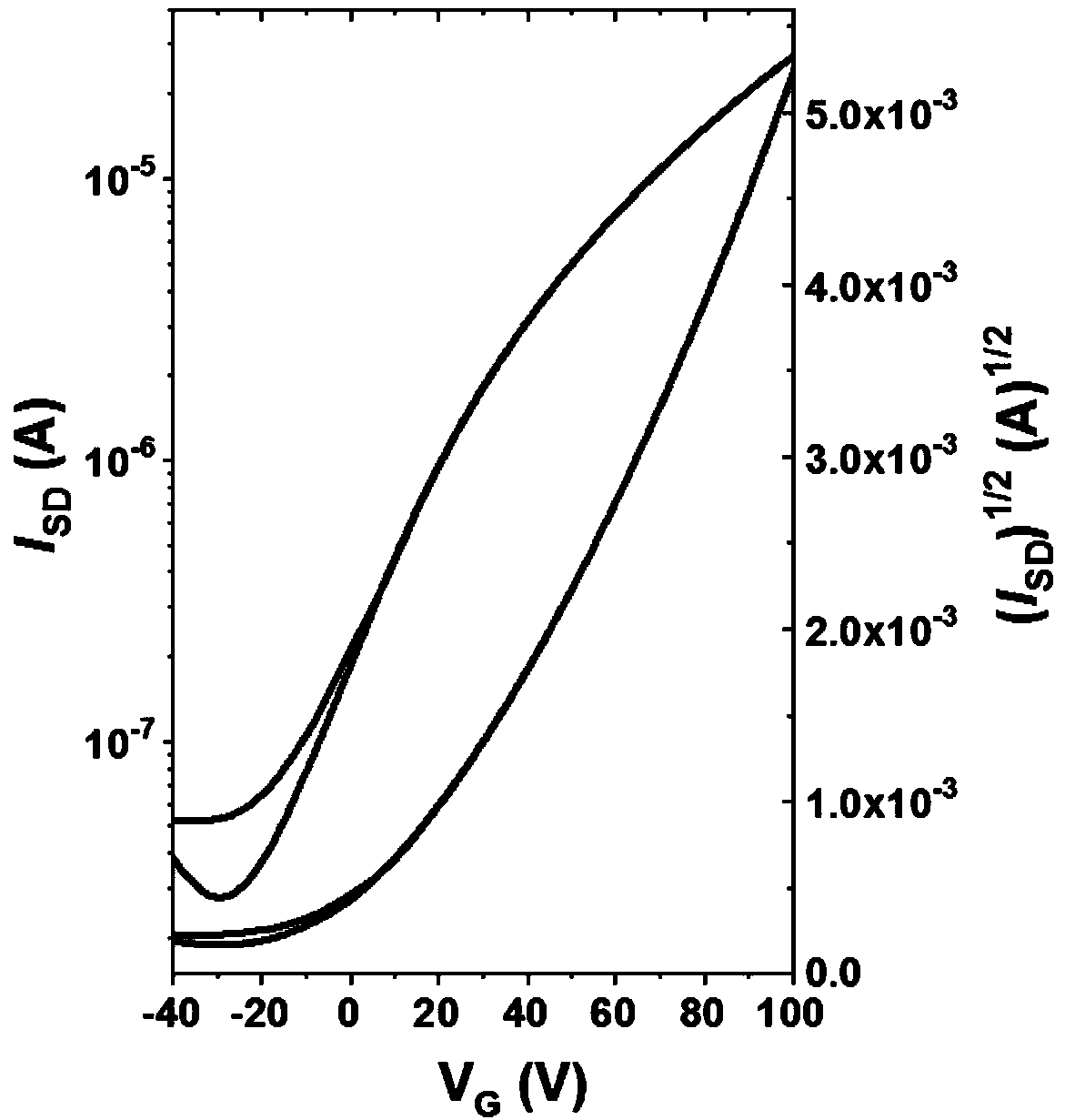
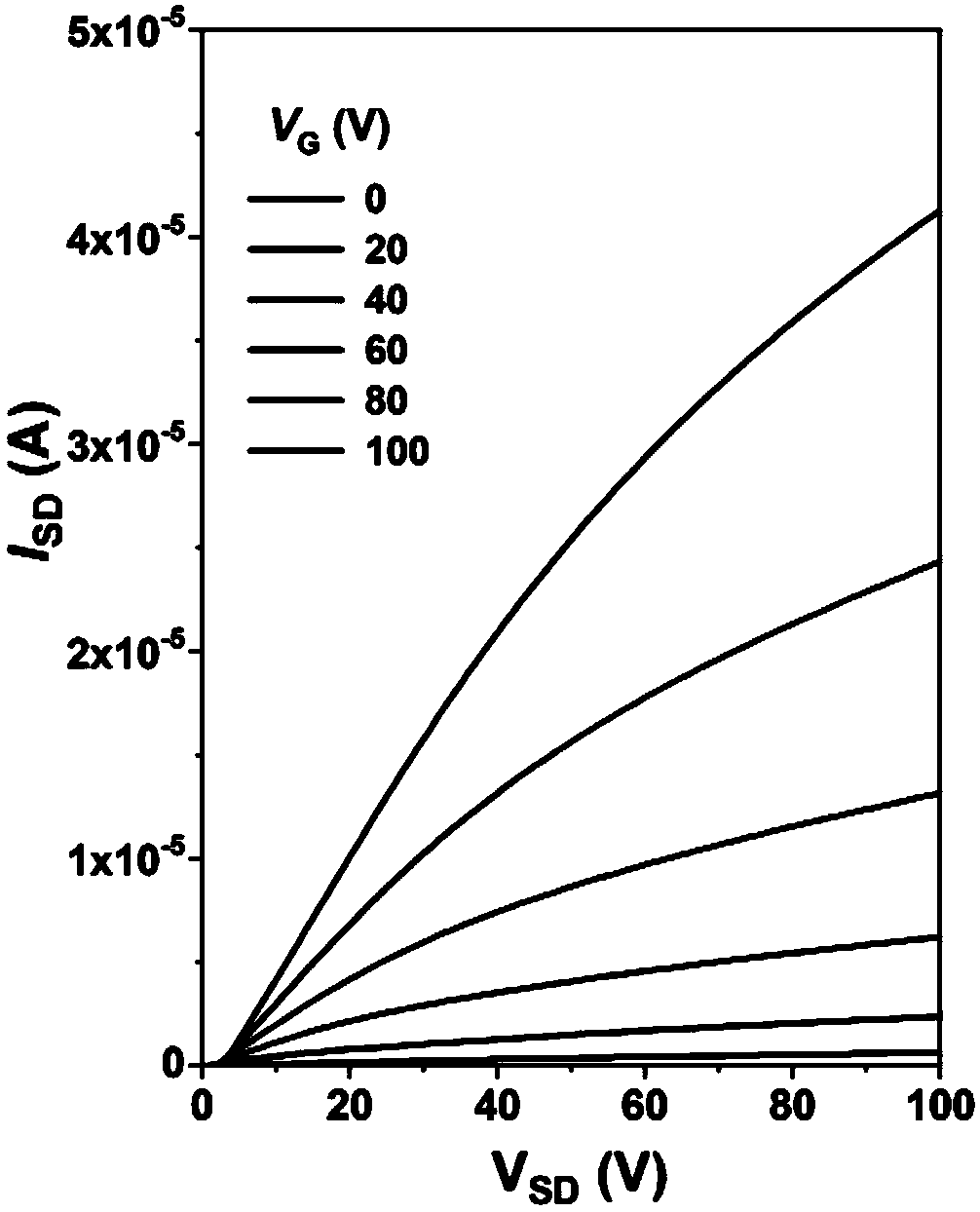
Rigid conjugated polymer based on benzodifurandione and derivatives thereof as well as preparation and application of rigid conjugated polymer
A technology of conjugated polymers and derivatives, applied in the field of organic functional materials and organic electronics, can solve the problems of n-type rigid conjugated polymers with less structure and poor performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Synthesis process of Compound 1: Add 2,5-dihydroxyterephthalic acid (1.0 g, 4.4 mmol) to dry toluene (50 mL), and then add acetic anhydride (10 mL). After mixing, they were heated to 100° C. for 5 hours, and then the solvent was removed under reduced pressure, and recrystallized from toluene to obtain a white solid (0.79 g, yield 94%). 1 H NMR (CDCl 3 ,400MHz,ppm):δ7.07(s,2H),3.78(s,4H). 13 C NMR (CDCl 3 ,100MHz,ppm): δ173.3,150.9,123.3,107.7,33.5.EI-MS(M + ) calc.: 190.03; found: 190.
Embodiment 2
[0078]
[0079] The synthesis process of compound 2: add benzodithiophene (0.45g, 2.34mmol) to dry tetrahydrofuran (40mL), cool down to -78°C, slowly add n-butyllithium (1.6M n-hexane solution, 5.85mL, 9.36mmol), and after stirring for 2 hours, tributylboronate (3.2mL, 12mmol) was added, returned to room temperature, and stirred for 8 hours. Add 100 mL of 1M hydrochloric acid to quench the reaction, filter, dissolve the filter residue with sodium hydroxide solution, and then add 1M hydrochloric acid until the solid no longer precipitates. Filter and dry the solid (0.49g, 80% yield) for the next reaction.
Embodiment 3
[0081]
[0082] The synthesis process of compound 3: Add compound 2 (0.51 g, 1.80 mmol) to tetrahydrofuran (50 mL), then add 1 mL of hydrogen peroxide (30%) solution, stir at room temperature for 1 hour, remove the solvent to obtain a solid, wash with water and methanol respectively, Compound 3 (0.3 g, yield 60%) was obtained. 1 H NMR (CDCl 3 ,400MHz,ppm):δ7.29(s,2H),3.99(s,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com