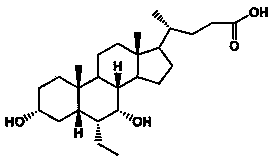
Preparation method for obeticholic acid liposome tablets
A technology of obeticholic acid lipid and obeticholic acid lipid, which is applied in the field of preparation of obeticholic acid liposome tablets, and can solve the problem of increasing differences in drug efficacy, affecting product efficacy, and large fluctuation range of tablet content. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
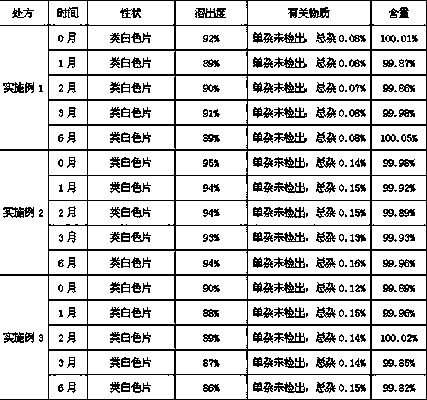
[0034] Preparation prescription of obeticholic acid liposome tablet:
[0035] Obeticholic Acid 5g
[0036] Lecithin 35g
[0037] Cholesterol 20g
[0038] Microcrystalline Cellulose 15g
[0039] Croscarmellose Sodium 1g
[0040] povidone k30 1g
[0041] Magnesium stearate 0.5g
[0042] Makes 1000 pieces
[0043] Its preparation method comprises the following steps:
[0044] Dissolve obeticholic acid in 20ml of ethanol, lecithin and cholesterol in 100ml of ethanol and chloroform (1:1) mixed solvent, then mix the two solutions evenly, heat and stir at 60°C for 2 hours, and then use a rotary evaporator The organic solvent was removed to obtain obeticholic acid liposomes, which were pulverized and passed through an 80-mesh sieve.
[0045] Mix the prepared obeticholic acid liposomes, microcrystalline cellulose, and croscarmellose sodium evenly, add 5% povidone K30 solution, make wet granules, dry, granulate, add stearin Magnesium acid mixed, pressed into tablets.
Embodiment 2
[0047] Preparation prescription of obeticholic acid liposome tablet:
[0048] Obeticholic Acid 10g
[0049] Lecithin 70g
[0050] Cholesterol 40g
[0051] Microcrystalline Cellulose 30g
[0052] Lactose 20g
[0053] Low-substituted hydroxypropyl cellulose 2g
[0054] Hypromellose 2g
[0056] Makes 1000 pieces
[0057] Its preparation method comprises the following steps:
[0058] Dissolve obeticholic acid in 40ml of ethanol, lecithin and cholesterol in 200ml of ethanol and chloroform (1:1) mixed solvent, then mix the two solutions evenly, heat and stir at 60°C for 2 hours, and then use a rotary evaporator The organic solvent was removed to obtain obeticholic acid liposomes, which were pulverized and passed through an 80-mesh sieve.
[0059] Mix the prepared obeticholic acid liposomes, microcrystalline cellulose, lactose, and low-substituted hypromellose evenly, add 5% hypromellose solution, make wet granules, dry, granulate, add hard ...
Embodiment 3
[0061] Preparation prescription of obeticholic acid liposome tablet:
[0062] Obeticholic Acid 25g
[0063] Lecithin 150g
[0064] Cholesterol 75g
[0065] Microcrystalline Cellulose 70g
[0066] Mannitol 50g
[0067] Crospovidone 5g
[0068] povidone k30 5g
[0069] Magnesium Stearate 2.5g
[0070] Makes 1000 pieces
[0071] Its preparation method comprises the following steps:
[0072] Dissolve obeticholic acid in 100ml of acetone, lecithin and cholesterol in 500ml of ethanol and chloroform (1:1) mixed solvent, then mix the two solutions evenly, heat and stir at 60°C for 2 hours, and then use a rotary evaporator The organic solvent was removed to obtain obeticholic acid liposomes, which were pulverized and passed through an 80-mesh sieve.
[0073]Mix the prepared obeticholic acid liposomes, microcrystalline cellulose, mannitol, and crospovidone evenly, add 5% povidone K30 solution, make wet granules, dry, granulate, add stearin Magnesium acid mixed, pressed into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com