Probe as well as synthesis method and application thereof
A synthesis method and probe technology, applied in material separation, instruments, analysis materials, etc., can solve problems such as damage to protein complexes, the study of interactions that cannot be used, and the study of interactions between proteins that cannot be directly applied, and achieves easy operation. , Improve enrichment and identification ability, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
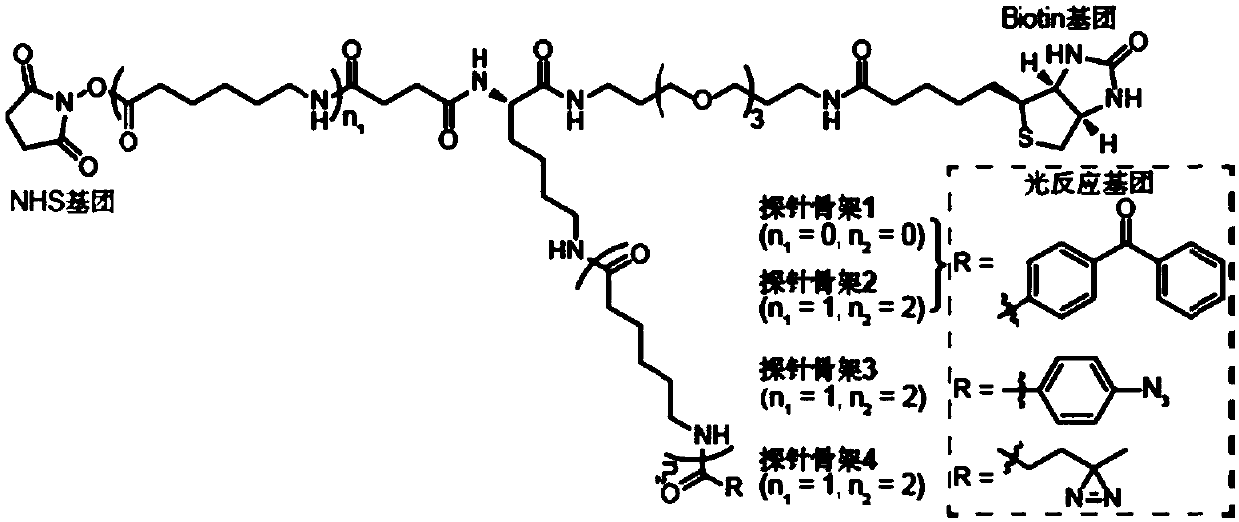
[0064] The synthesis of embodiment 1 probe skeleton 1
[0065]
[0066] According to literature [Ouchi T, Yamabe E, Hara K, Hirai M, Ohya Y (2004) Design of attachment type of drug delivery system by complex formation of avidin with biotinyl drug model and biotinyl saccharide. J Control Release 94 (2-3): 281 -291; Frei AP, Wollscheid B, Jeon OY, Carreira EM US 2014 / 0011212A1] Synthesis of compound c1;
[0067] A mixture of c1 (896 mg, 1 mmol) and diethylamine (2.19 g, 30 mmol) in acetonitrile was stirred at room temperature, and when the starting material c1 disappeared (monitored by TLC), the solvent was removed; the residue was dissolved in dimethylformamide (DMF , 10 mL), added N,N-diisopropylethylamine (258 mg, 2 mmol) and succinic anhydride (200 mg, 2 mmol), stirred for more than 12 hours, removed DMF, separated the residue by silica gel, and obtained in 50% yield c2 (387 mg);
[0068] HRMS (m / z): [M-H] - C 35 h 61 o 11 N 6 The calculated value of S is 773.4125,...
Embodiment 2
[0075] The synthesis of embodiment 2 probe skeleton 2
[0076]
[0077]According to literature [Ouchi T, Yamabe E, Hara K, Hirai M, Ohya Y (2004) Design of attachment type of drug delivery system by complex formation of avidin with biotinyl drug model and biotinyl saccharide. J Control Release 94 (2-3): 281 -291; Frei AP, Wollscheid B, Jeon OY, Carreira EM US 2014 / 0011212A1; Srinivasan B, Huang X (2008) Functionalization of magnetic nanoparticles with organic molecules: loading level determination and evaluation of linker length effect on immobilization. Chirality 20( 4): 265-277] synthesis of compound c4;
[0078] At 0°C, TFA (1.026g, 9mmol) was added to a solution of c4 (100mg, 0.09mmol) in dichloromethane (5mL), stirred for 2 hours, TLC monitoring found that the raw material c4 disappeared; after the mixture was evaporated in vacuo, the dissolved In DMF (5 mL), triethylamine was added to adjust the pH to greater than 9; 4-benzoylbenzoic acid-2,5-dioxopyrrolidin-1-yl est...
Embodiment 3
[0084] Synthesis of Example 3 Probe Skeleton 3
[0085]
[0086] At 0°C, TFA (1.026g, 9mmol) was added to a solution of c4 (100mg, 0.09mmol) in dichloromethane (5mL), stirred for 2 hours, TLC monitoring found that the raw material c4 disappeared; after the mixture was evaporated in vacuo, the dissolved In DMF (5 mL), add triethylamine to adjust the pH to greater than 9; add 2,5-dioxopyrrolidin-1-yl 4-azidobenzoate (35.2 mg, 0.14 mmol), and stir overnight , the solvent was removed in vacuo and the residue was separated through silica gel to afford c6 (63 mg) in 61% yield;
[0087] HRMS (m / z): [M-H] - C 55 h 89 o 13 N 12 The calculated value of S is 1157.6398, and the measured value is 1157.6417;
[0088] Add EDCI (30mg, 0.158mmol) to the DMF (5mL) mixed solution of compound c6 (61mg, 0.053mmol) and hydroxysuccinimide (12mg, 0.106mmol), stir at room temperature overnight, when compound c6 disappeared (by TLC monitoring), the solvent was removed under vacuum, the residu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com