Streptococcus pneumoniae vaccine and preparation method thereof
A Streptococcus pneumoniae and vaccine technology, applied in peptide preparation methods, botany equipment and methods, biochemical equipment and methods, etc., can solve the problems of limited serotypes, protective antibodies produced by infants and young children, and achieve the goal of disease prevention Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A Streptococcus pneumoniae antigen SPD_0151 protein, the amino acid sequence of which is SEQ ID NO.1.
[0031] Its preparation method comprises the following steps:
[0032] (1) Link the spd-0151 gene (its sequence is SEQ ID NO.2) without signal peptide sequence to the pGEX-4T-1 vector to obtain the expression vector pGEX-4T-0151;
[0033] (2) Introduce the expression vector pGEX-4T-0151 into Escherichia coli to obtain the expression strain; inoculate the expression strain in LB medium containing 100 ng / μL ampicillin, culture it with shaking at 37°C overnight, and continue to expand the culture; the strain to be expressed Proliferate until the OD600 in the logarithmic phase of growth is 0.6-0.8, add IPTG (isopropyl-β-D-thiogalactoside) to the culture system to a mass concentration of 0.5mmol / L, induce expression for 6h, and collect the bacteria Body, cleavage to obtain protein;
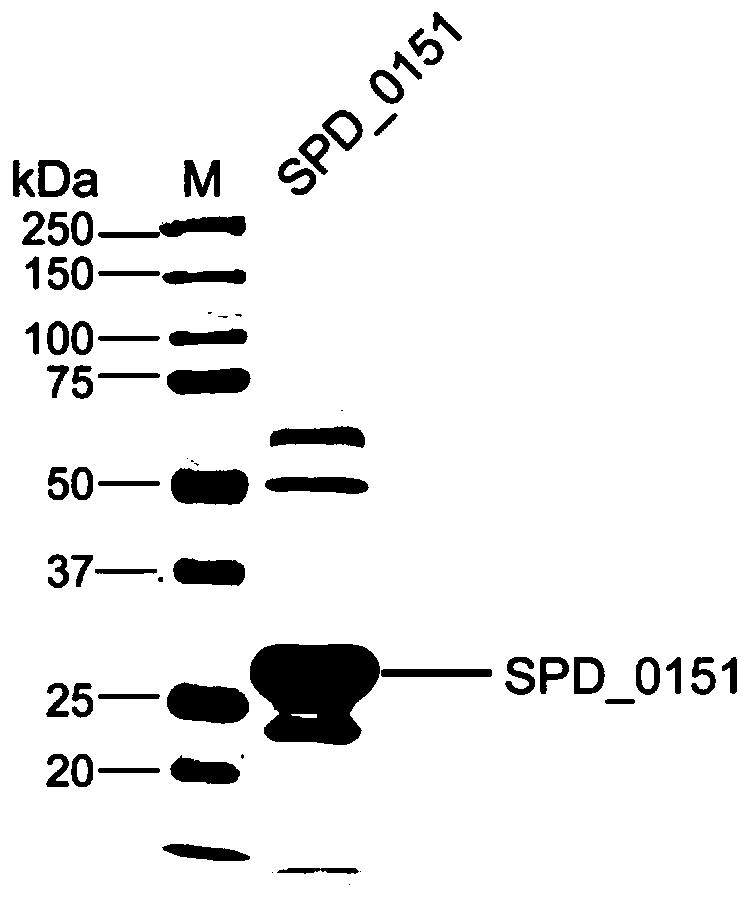
[0034] (3) GST affinity chromatography is used to purify the obtained protein to obtain t...
Embodiment 2
[0036] Experimental group: the mixture of the target protein SPD_0151 and aluminum adjuvant obtained in Example 1, the concentration of the target protein SPD_0151 in the mixture is 125 μg / mL, the concentration of the aluminum adjuvant is 0.5 mg / mL, and the composition of the aluminum adjuvant used is Aluminum hydroxide;
[0037] Control group: a mixture of 1×PBS and aluminum adjuvant, the concentration of the aluminum adjuvant in the mixture is 0.5 mg / mL, wherein the component of the aluminum adjuvant used is aluminum hydroxide.
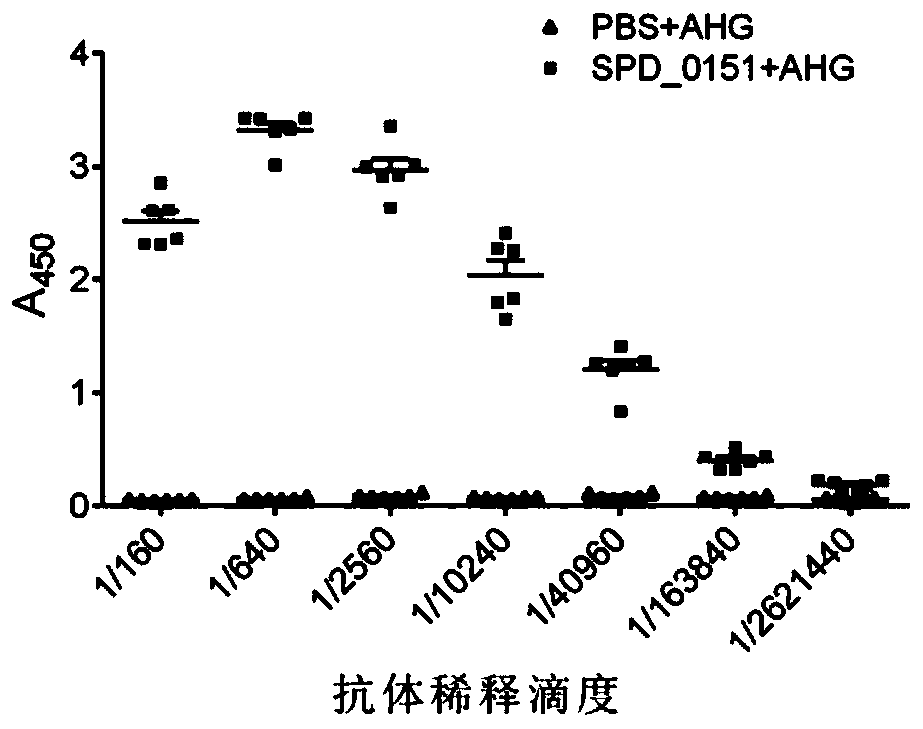
[0038] The above-mentioned experimental group and control group were used to subcutaneously inject BALB / c mice at multiple sites, the injection amount was 25 μg / mouse, and 10 mice in each group were immunized three times with an interval of 14 days between each immunization. Seven days after the completion of the third immunization, the antibody titer in the mouse serum was determined by enzyme-linked immunosorbent assay.
[0039] Usually, the immu...
Embodiment 3
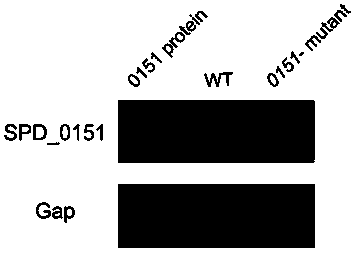
[0042] The serum (antibody containing SPD_0151 protein) of the mice immunized by the experimental group in Example 2 was used as the primary antibody, and was detected by Western blot (Western blot).
[0043] Such as image 3 As shown, the antibody in the mouse serum can specifically recognize the SPD_0151 protein, but the mutant of the SPD_0151 protein cannot be detected, and the detection result is a single band, indicating that the antibody has good specificity; and the antibody can not only be used to detect prokaryotic expression Purified SPD_0151 protein, and can detect SPD_0151 protein expressed in Streptococcus pneumoniae, wherein the internal reference is Gap antibody (GAPDH, purchased from Beijing Quanshijin Biotechnology Co., Ltd.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com