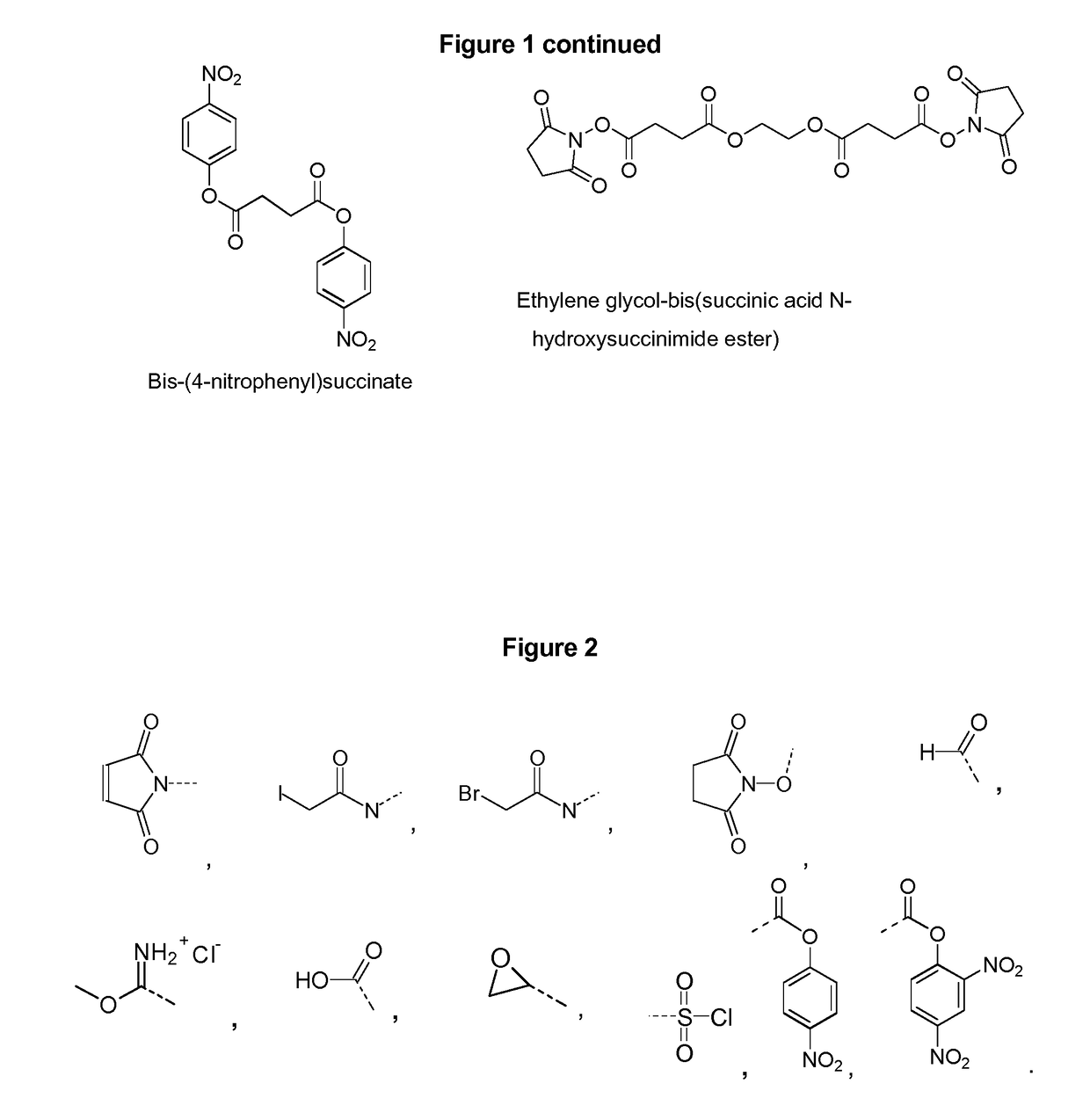
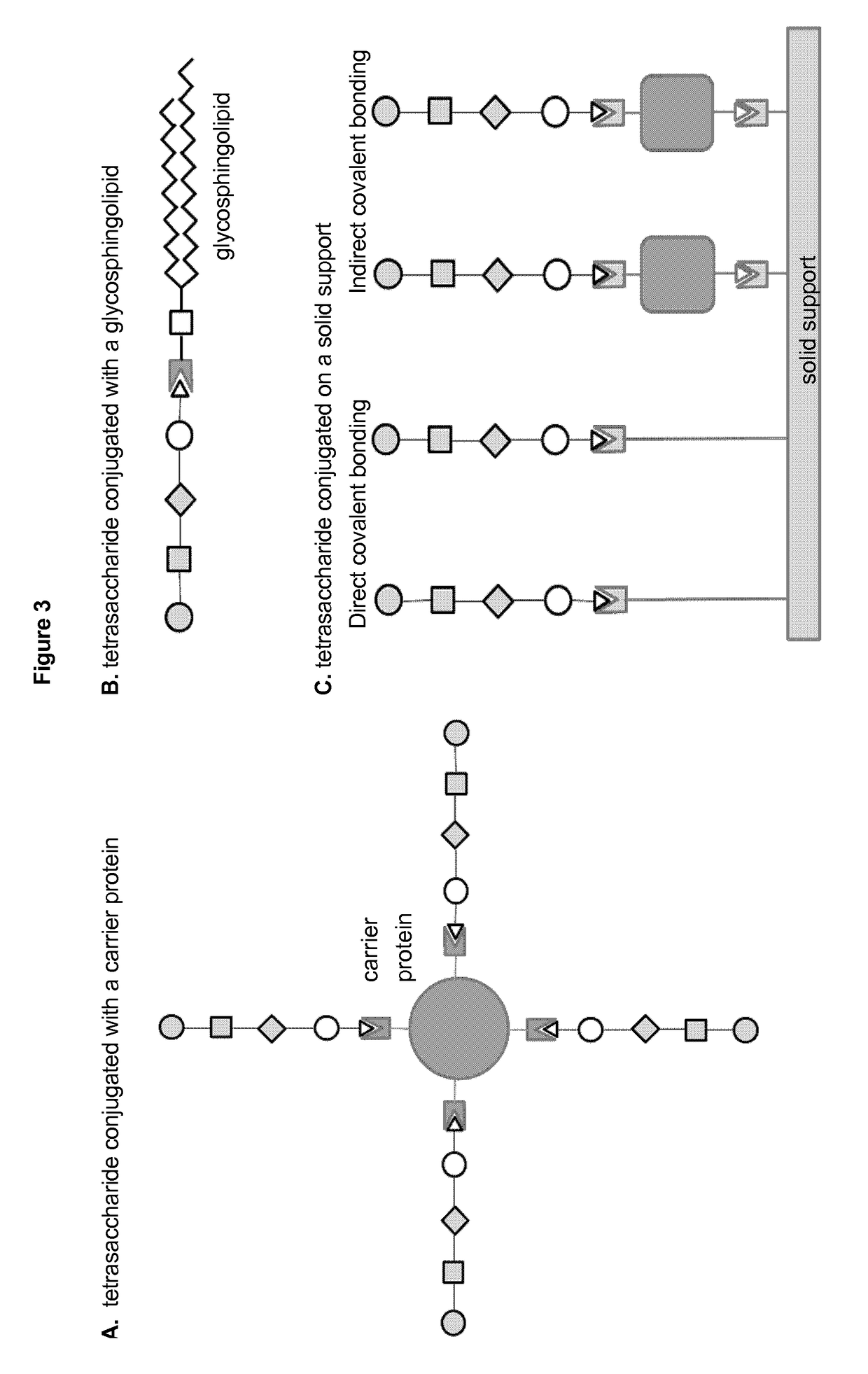
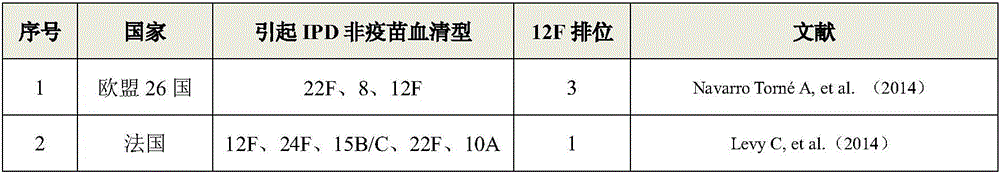
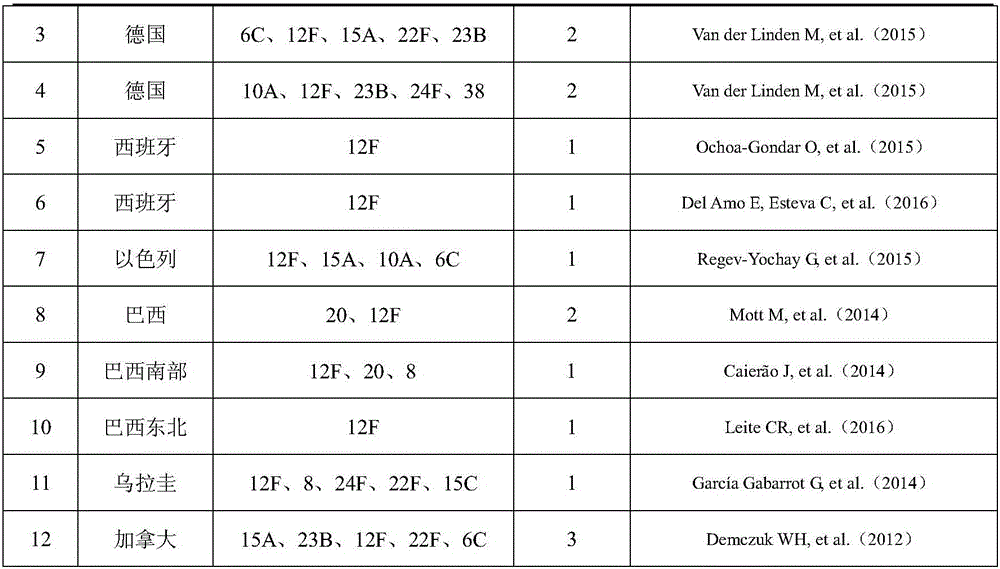
Patents
Literature
36 results about "Streptococcus pneumoniae bacteria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pneumonia can be caused by a variety of viruses, bacteria, and sometimes fungi. Pneumococcal pneumonia is caused by bacteria called Streptococcus pneumoniae or strep. S. pneumoniae is also called pneumococcus.
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
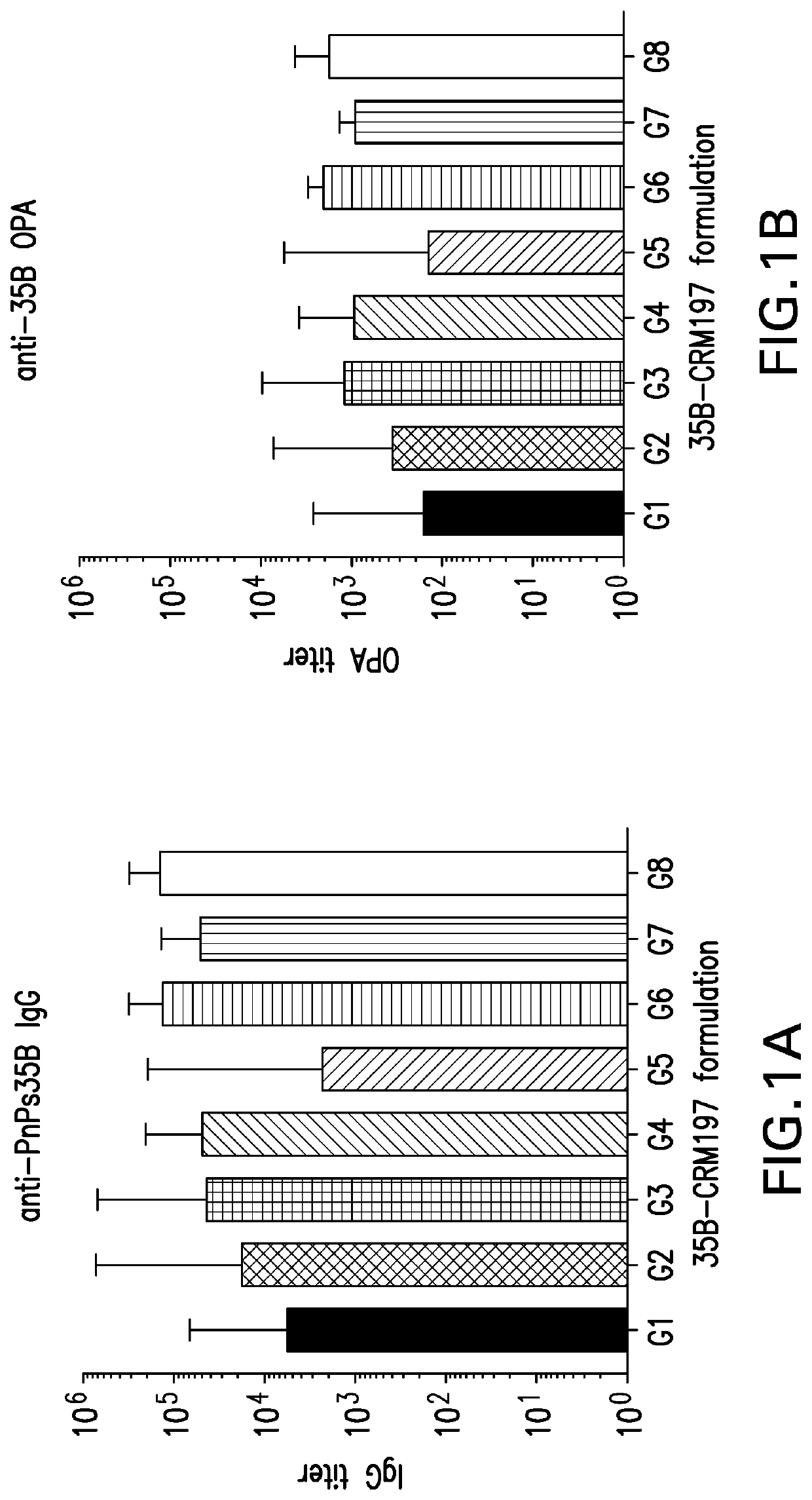
Immunogenic Compositions for Use in Pneumococcal Vaccines
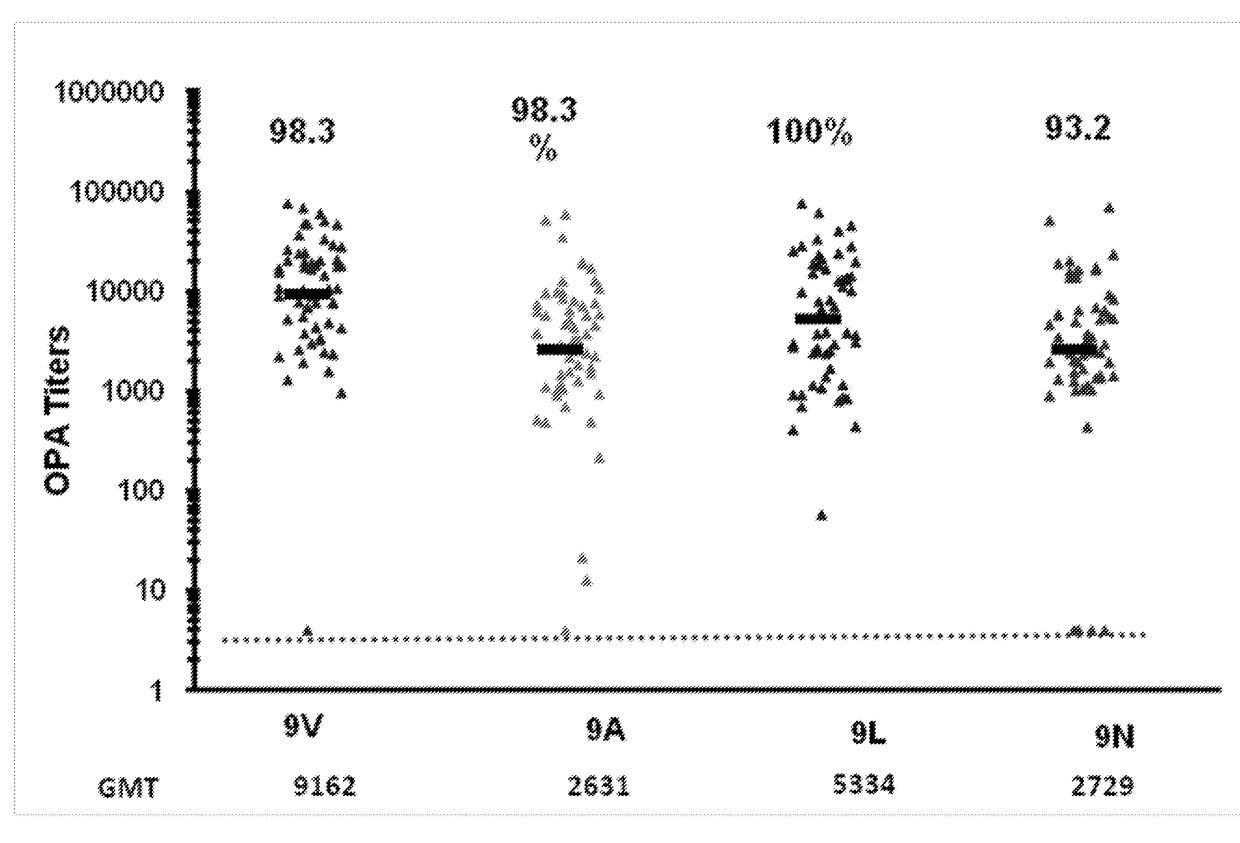
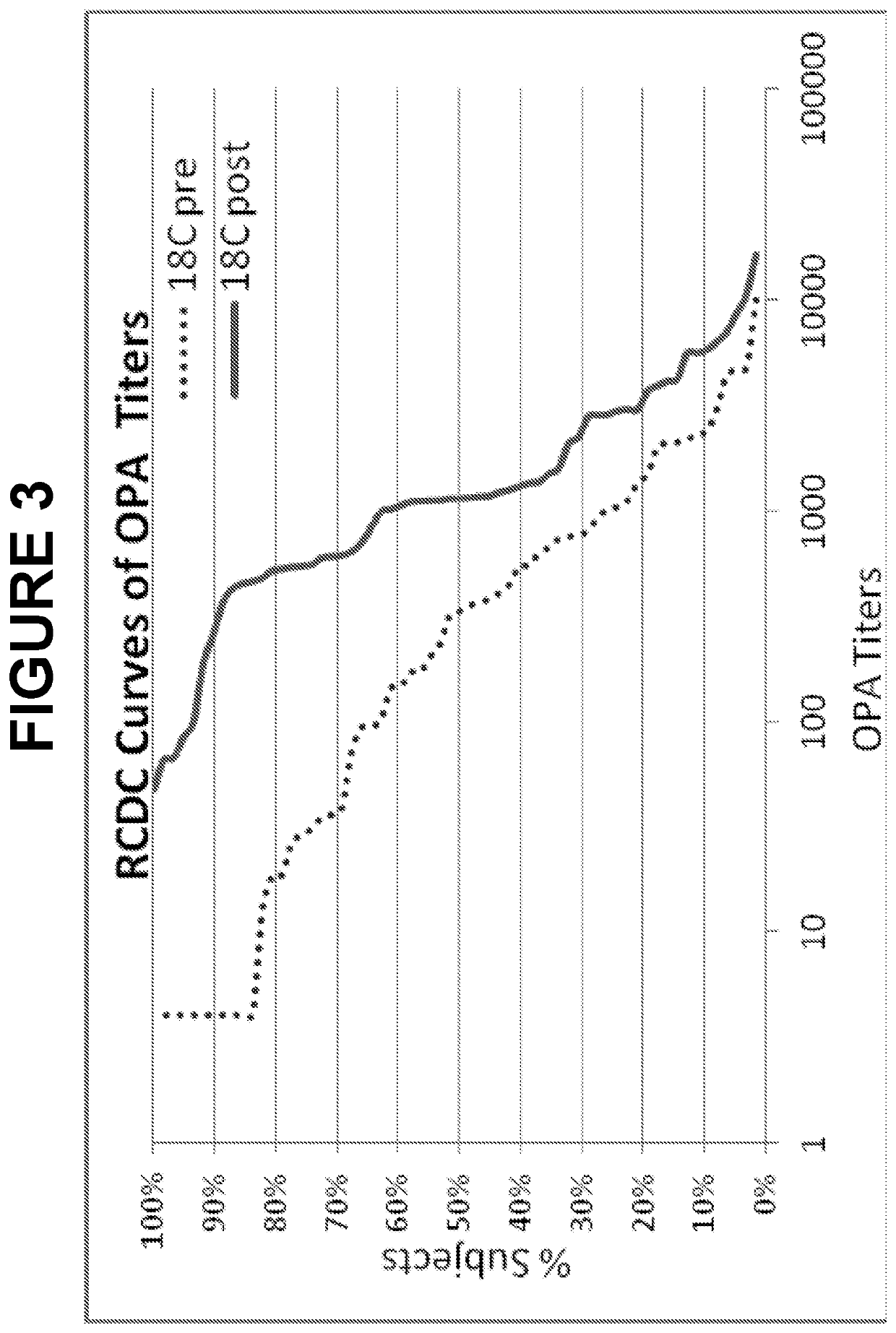
ActiveUS20180000922A1Raise the ratioIncrease OPA titerAntibacterial agentsBacterial antigen ingredientsPneumococcal vaccineImmunogenicity
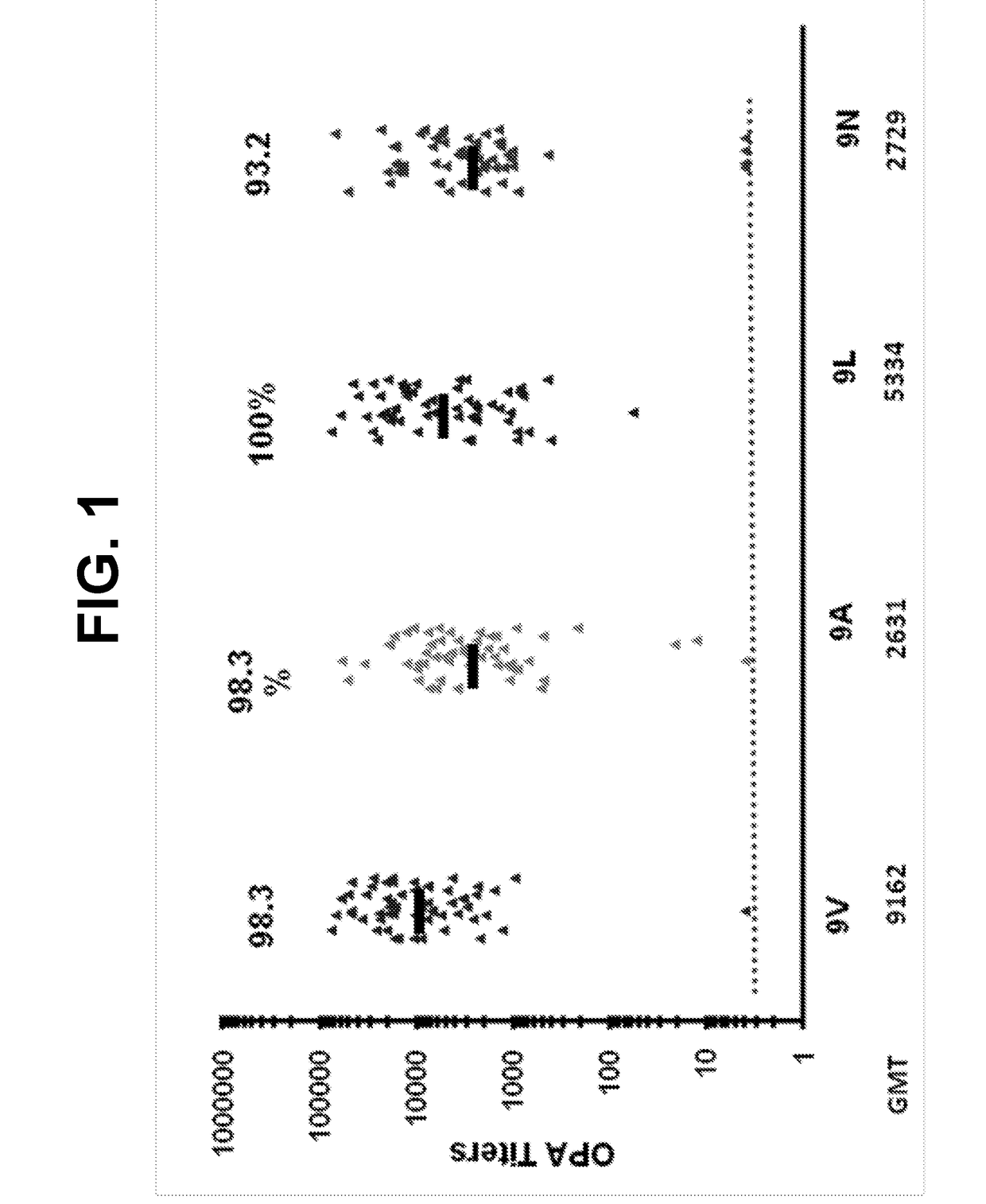
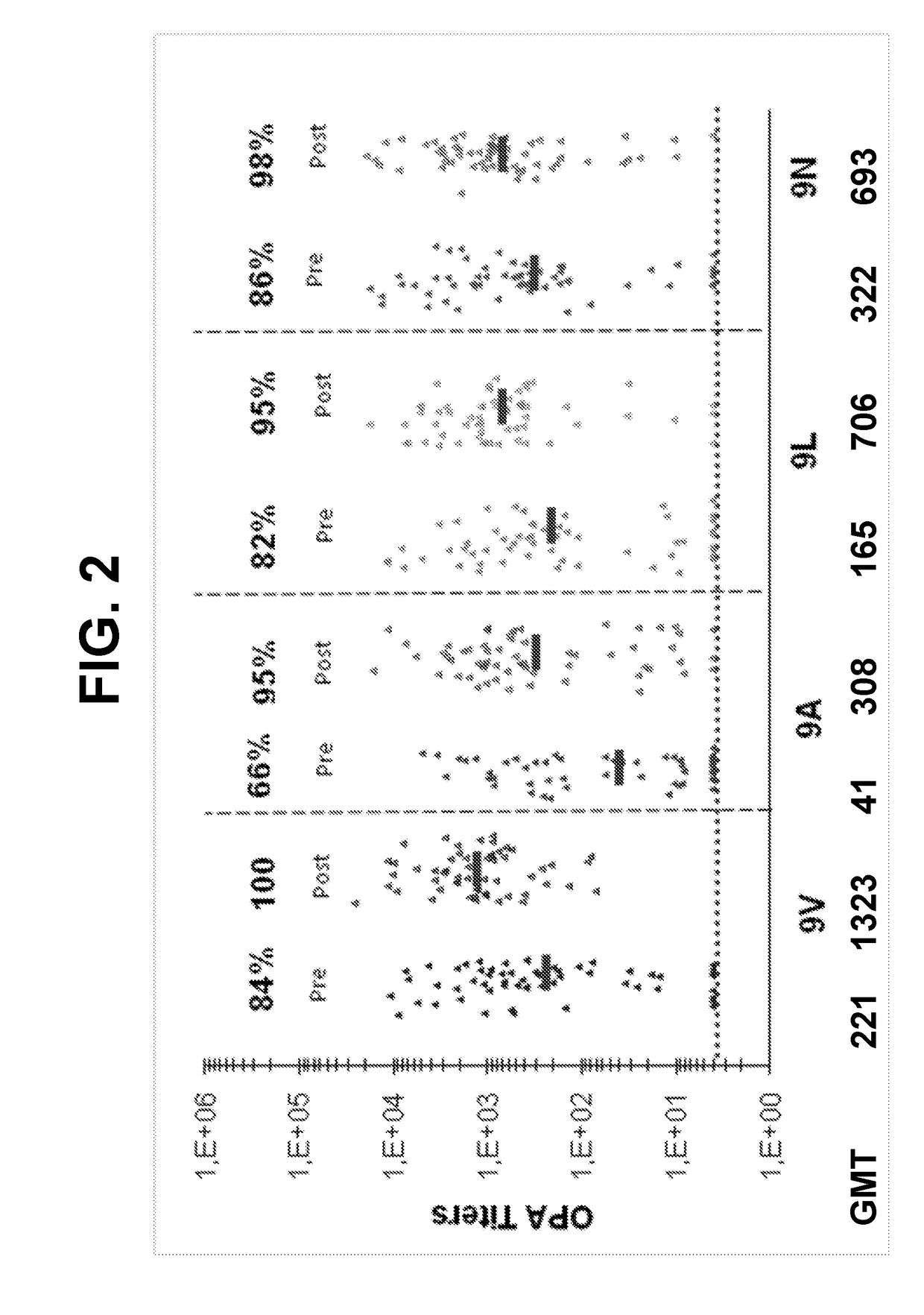
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 9, while limiting the number of conjugates. The present invention thereforerelates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Multiple vaccination including Serogroup C Meningococcus
ActiveUS20100034847A1Accurate measurementAvoid potential suppression effectAntibacterial agentsBacterial antigen ingredientsVaccinationAdjuvant
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminium phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS20130023443A1Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
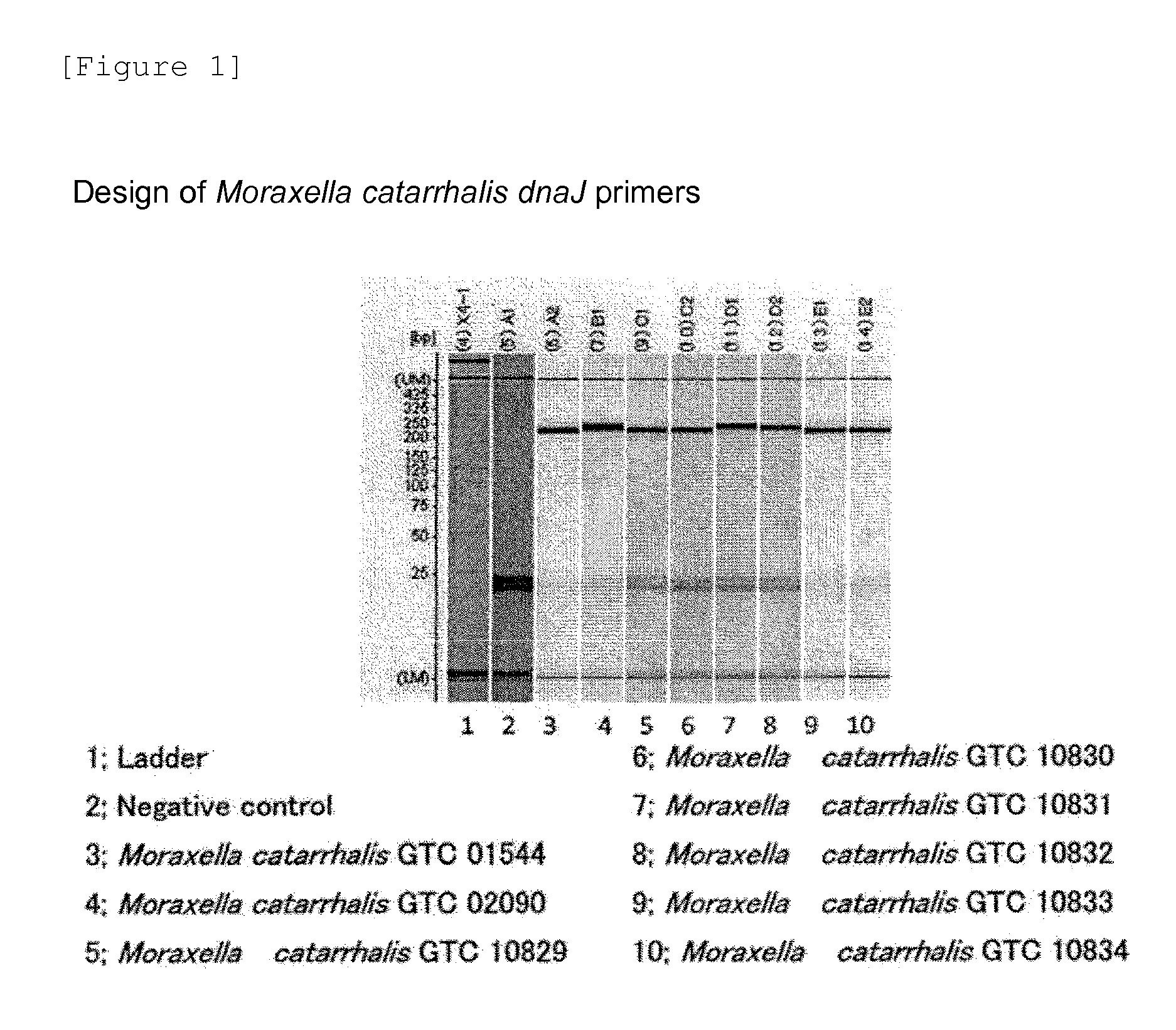
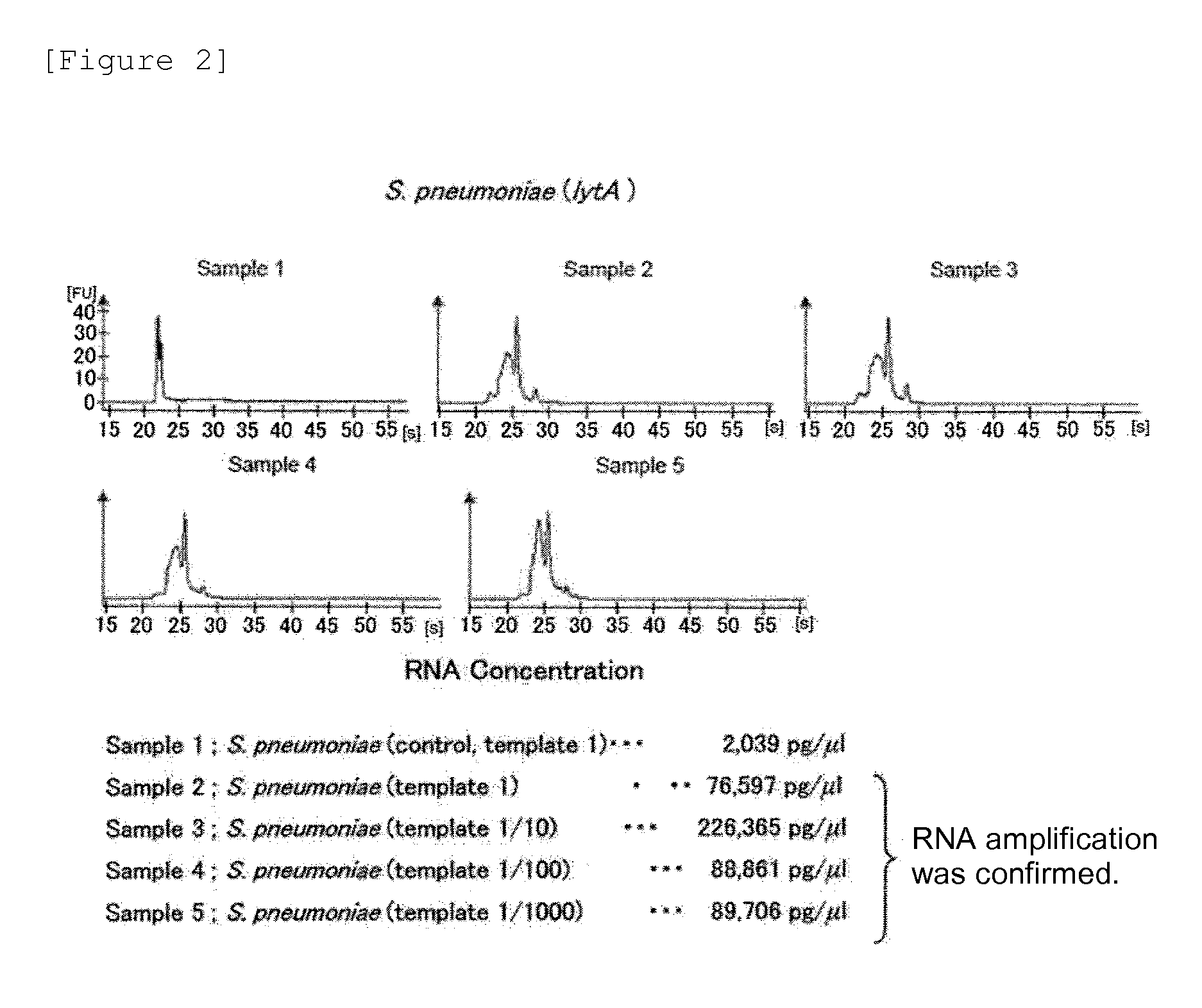
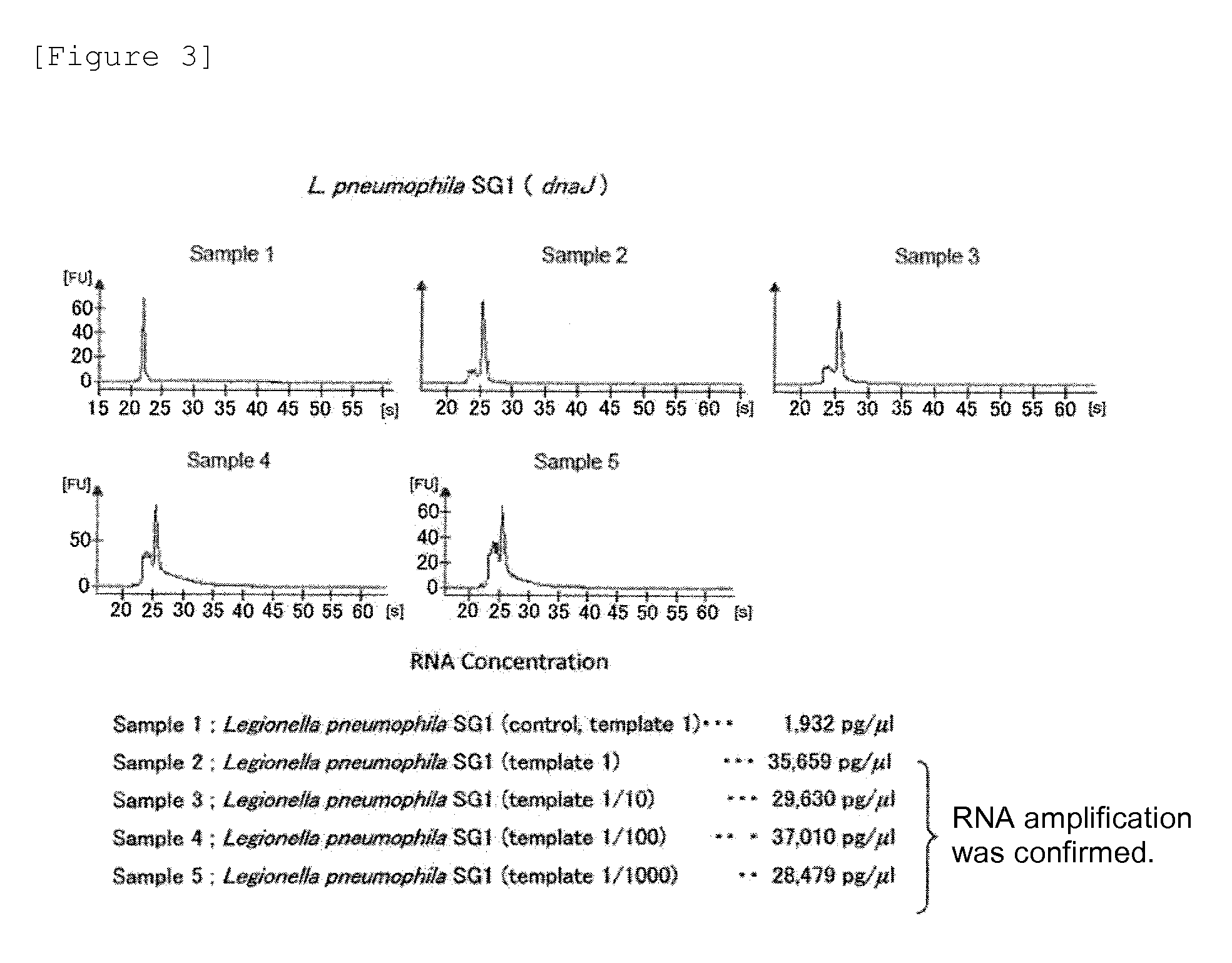
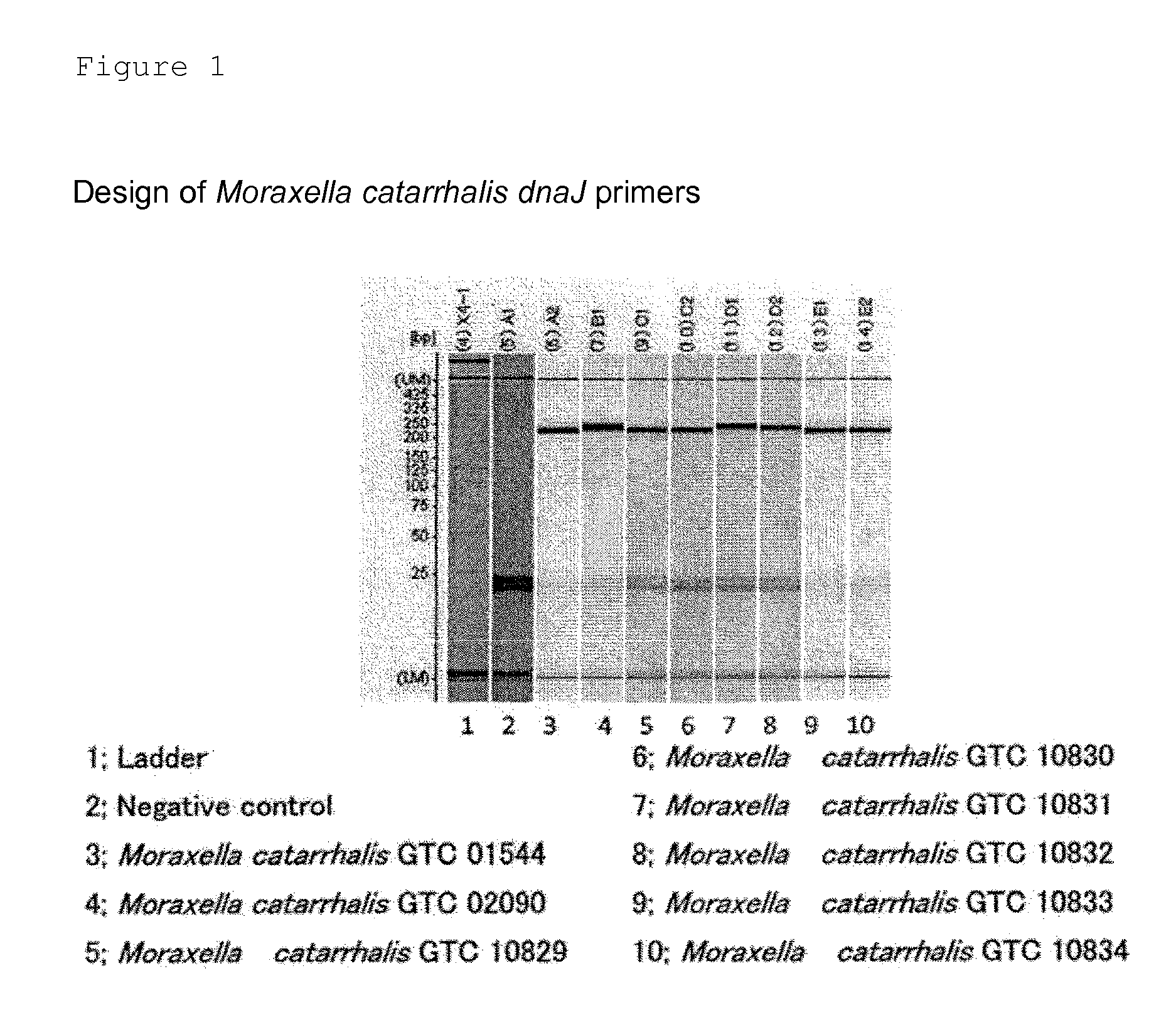
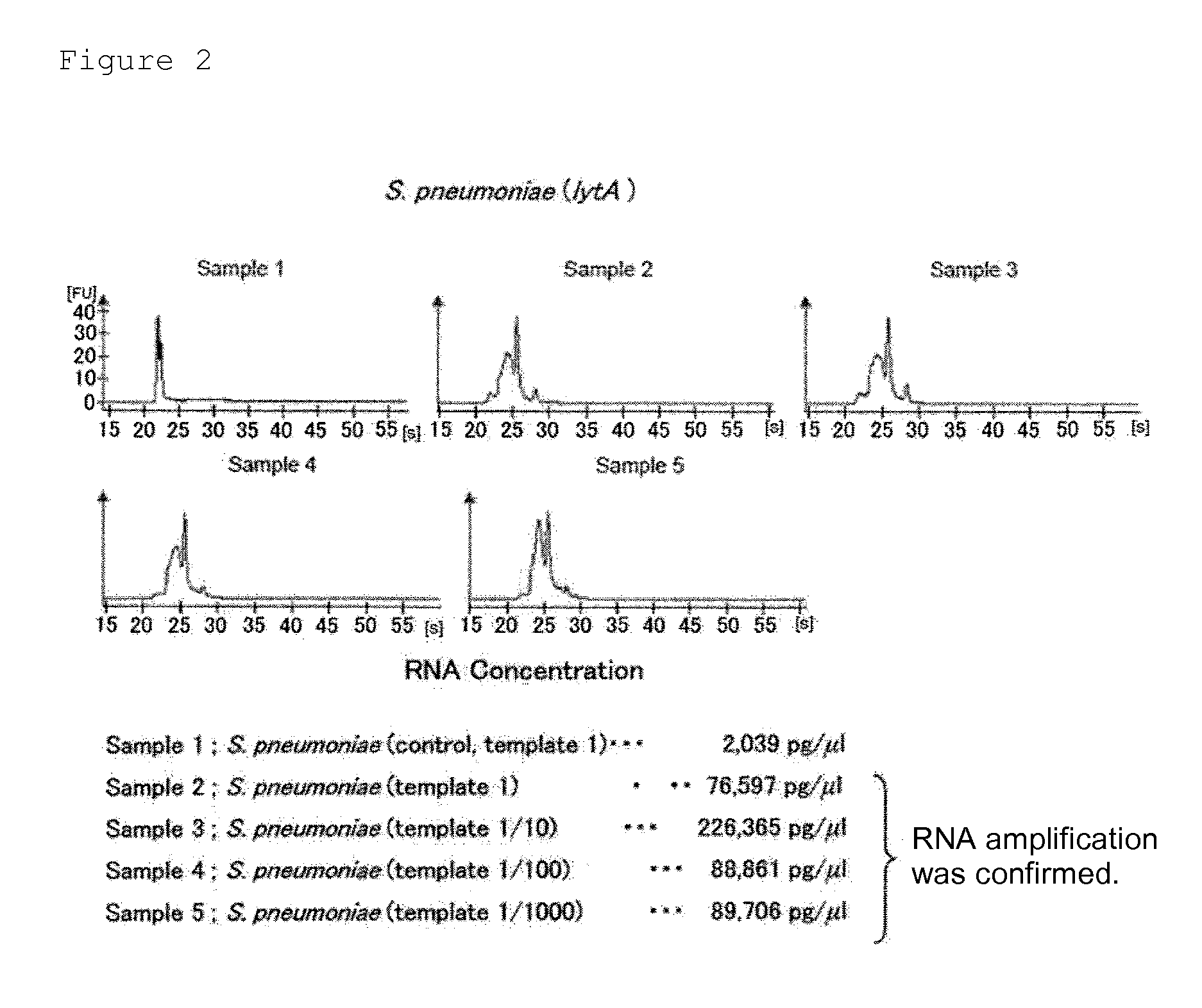
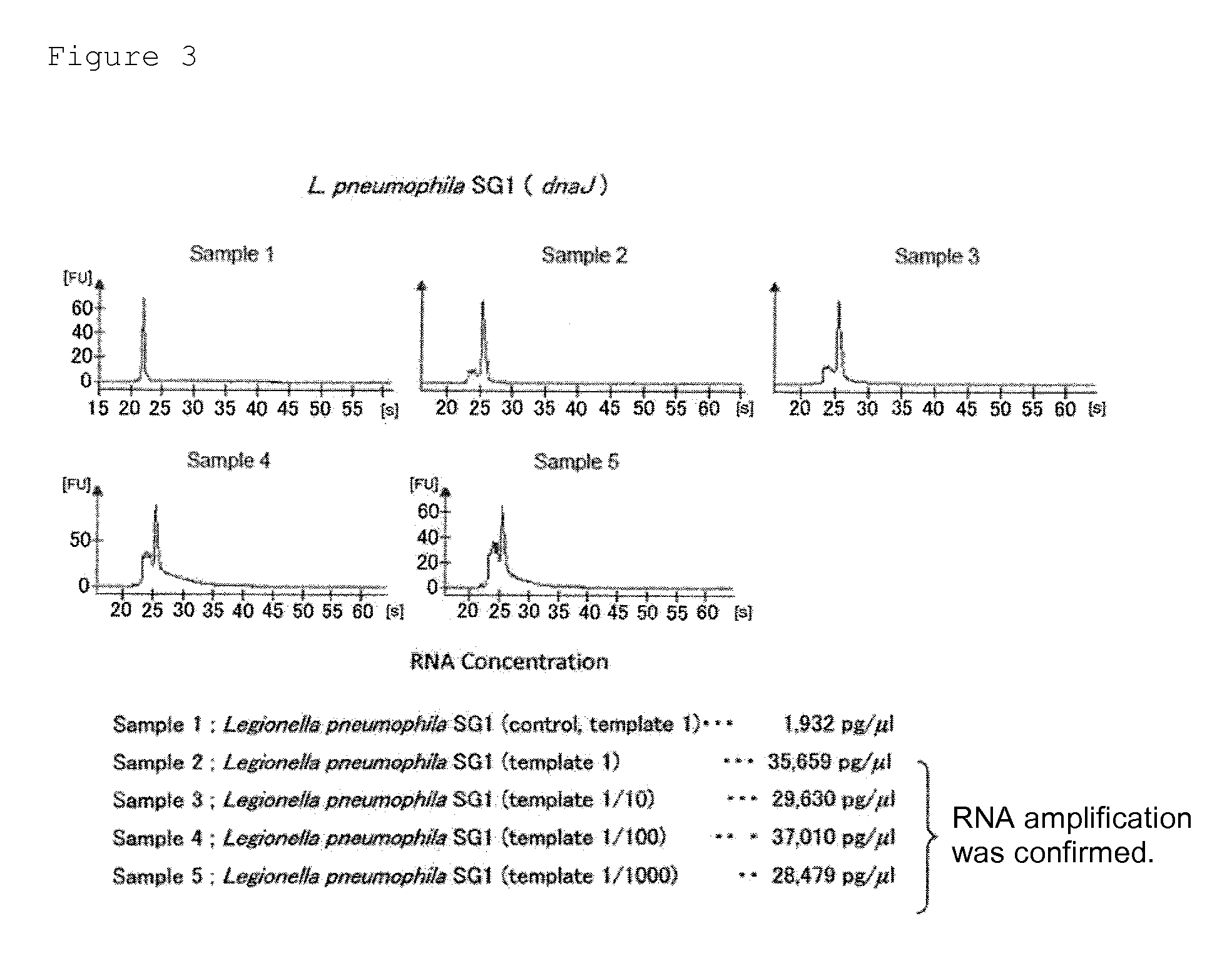
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
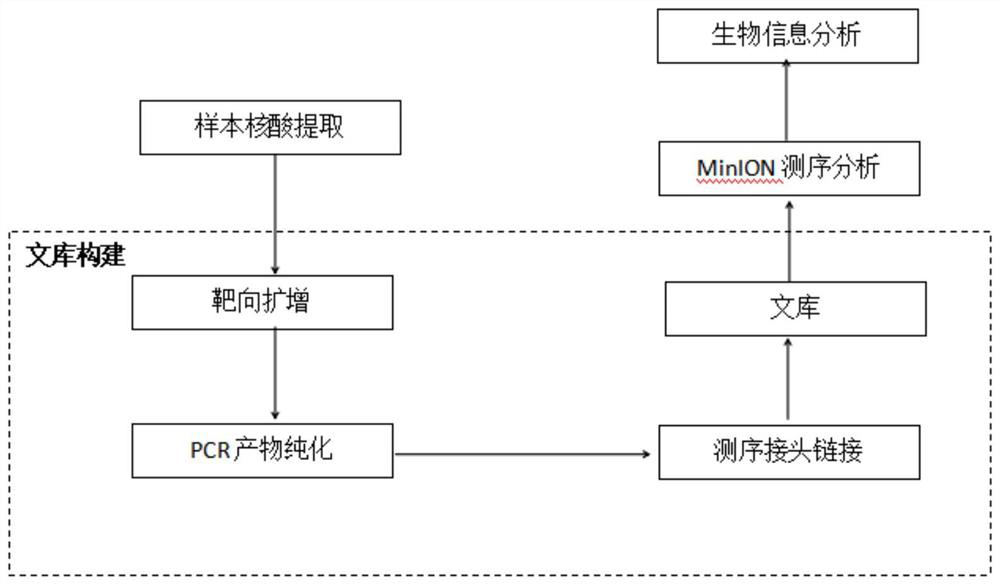
Primer group and kit for rapidly identifying respiratory tract microorganisms based on nanopore sequencing and application of primer group
ActiveCN112501268APrecise positioningImprove accuracyMicrobiological testing/measurementAgainst vector-borne diseasesPneumonitisEnterococcus
The invention discloses a primer group for detecting respiratory tract microorganisms based on a nanopore sequencing method. The nucleotide sequences of the primer group are as shown in SEQ ID NO:1-20. The microorganisms are streptococcus pneumoniae, staphylococcus aureus (resisting to methicillin), klebsiella pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, stenotrophomonas maltophilia, candida albicans, haemophilus influenzae, legionella pneumophila, enterococcus faecium, chlamydia psittaci, cryptococcus gattii, aspergillus fumigatus and pneumocystis jiroveci. According to the invention, sequencing optimization is carried out on different types of samples of the respiratory tract microorganisms, so that the detection method, the kit and the like are suitable for various typesof respiratory tract samples; and a targeted amplification method is adopted, so that the detection requirements of sputum, alveolar lavage and other sample types can be met. In addition, parallel sequencing can be performed, so that the flux of the detected samples is increased, the sequencing time is shortened, and the contradiction between the flux of detected pathogenic species and the cost and time is further relieved.
Owner:GUANGZHOU DARUI BIOTECH
Vaccines against Streptococcus pneumoniae serotype 8
ActiveUS10220083B2Improving immunogenicityImprove flow characteristicsAntibacterial agentsOrganic active ingredientsProtection sexAntibody
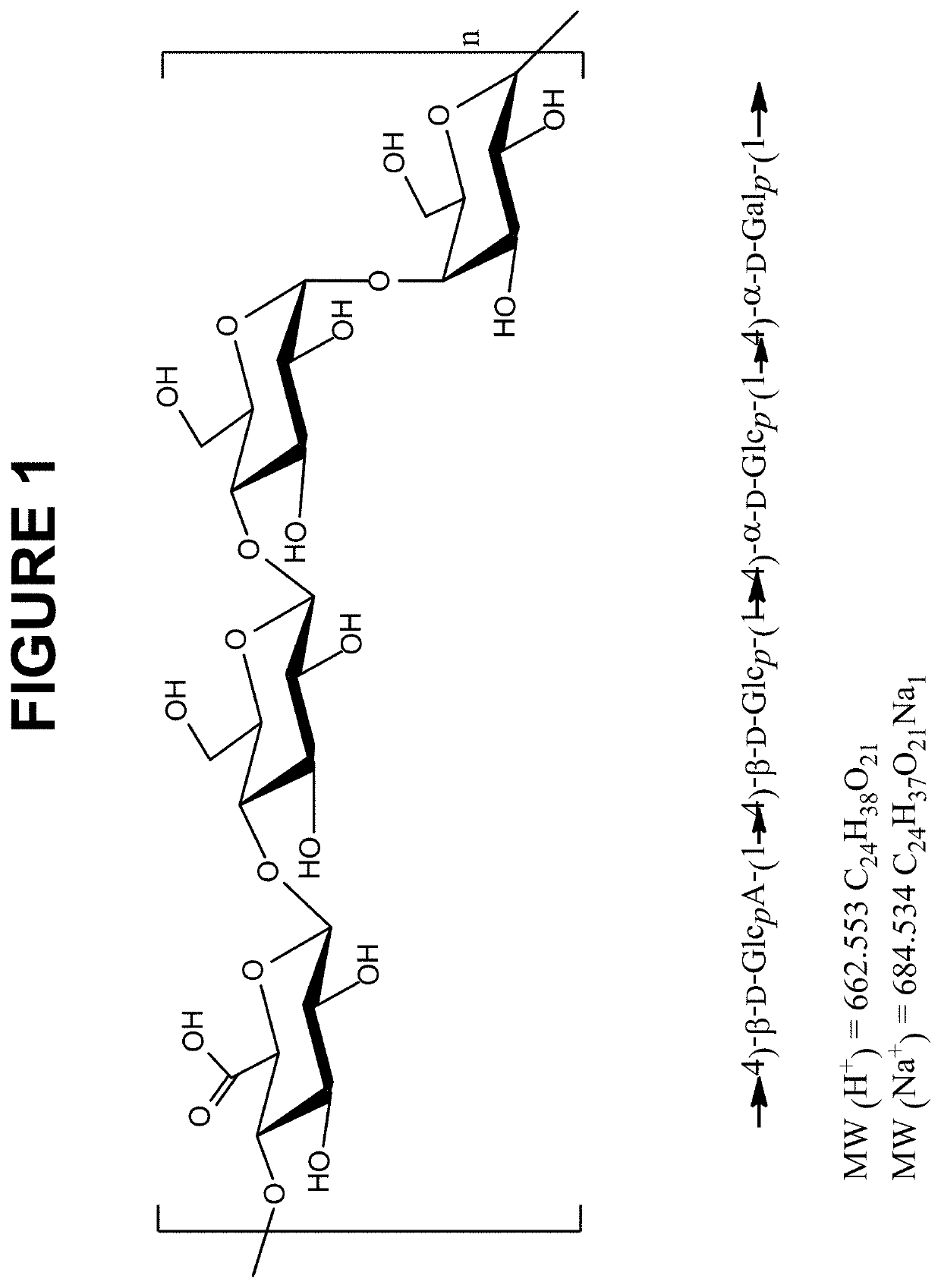
The present invention relates to synthetic saccharides of general formula (I) that are related to Streptococcus pneumoniae serotype 8 capsular polysaccharide, conjugates thereof and the use of said saccharides and conjugates for raising a protective immune response in a human and / or animal host. Furthermore, the synthetic saccharide structures of general formula (I) are useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
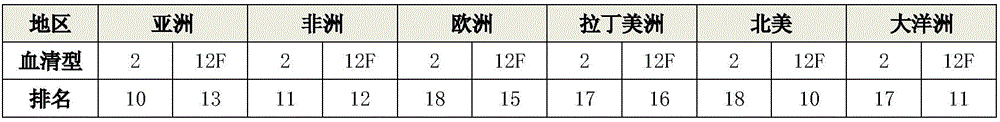
15-valence pneumococcus conjugate combined vaccine containing 2-type and 12 F serotypes
PendingCN105999254AGrowth assessment positive conversion rateAntibacterial agentsBacterial antigen ingredientsCoccidiaSerotype
The invention relates to a 15-valence pneumococcus conjugate combined vaccine. The combination particularly contains 2-type and 12 F serotypes, has a wide immunization coverage rate and is used for preventing infectious diseases caused by pneumococcus. The combined vaccine further contains a 1-type serotype, a 3-type serotype, a 4-type serotype, a 5-type serotype, a 6A serotype, a 6B serotype, a 7F serotype, a 9V serotype, a 14-type serotype, a 18 C serotype, a 19 A serotype, a 19 F serotype and a 23 F serotype.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
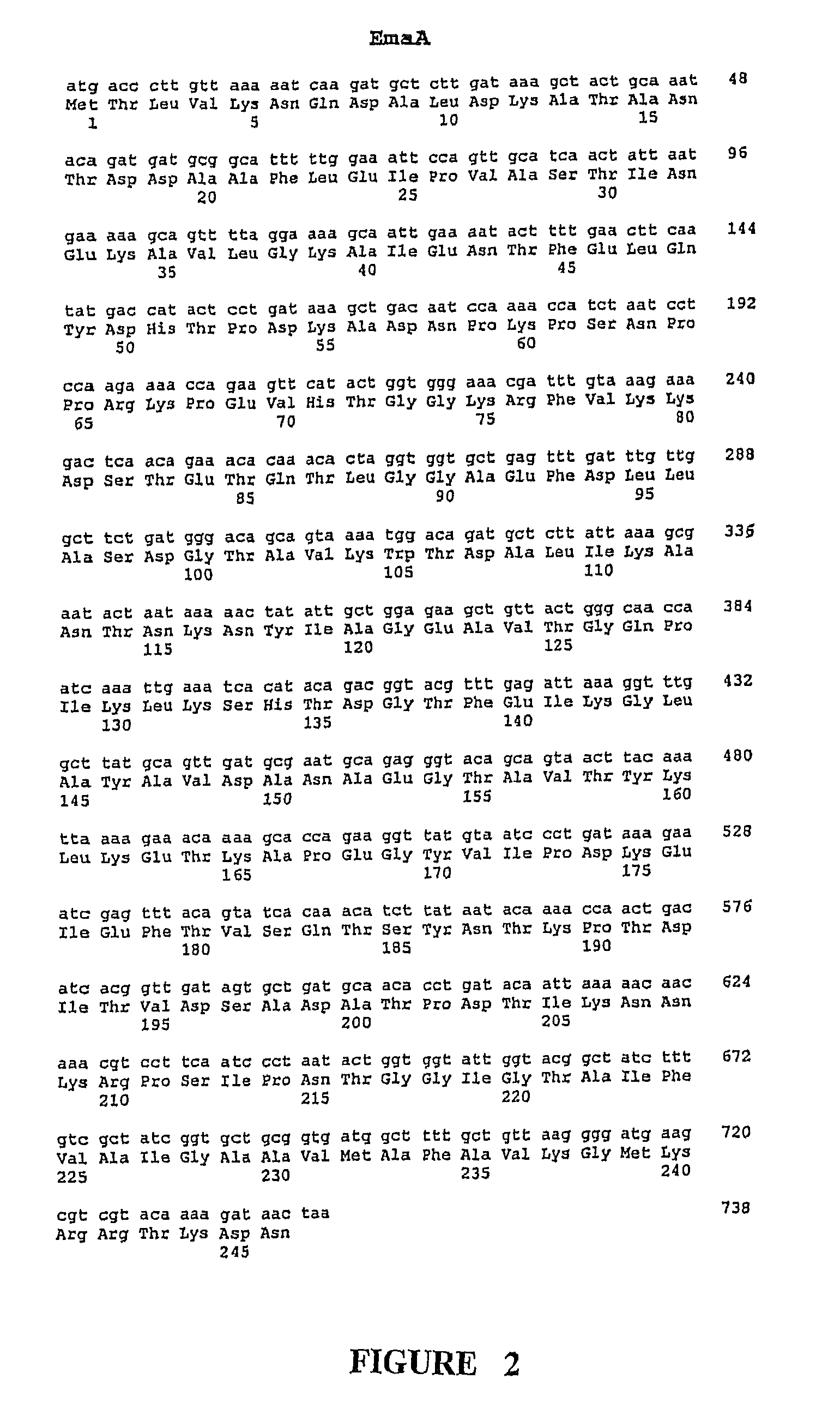
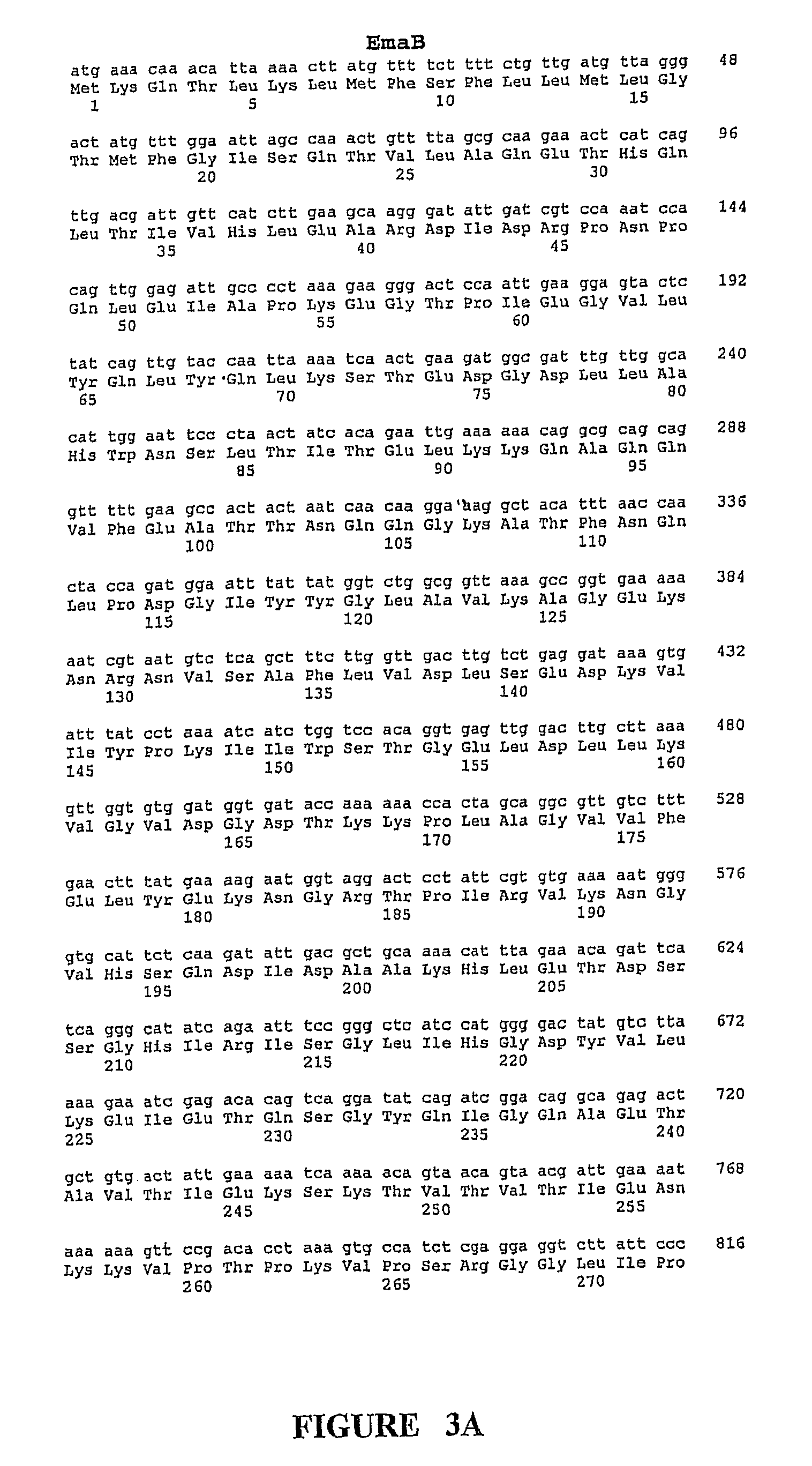
Group B Streptococcus polypeptides nucleic acids and therapeutic compositions and vaccines thereof
InactiveUS7892552B2Avoid infectionAntibacterial agentsBacterial antigen ingredientsMutantStreptococcus mitis
This invention provides isolated nucleic acids encoding polypeptides comprising amino acid sequences of streptococcal matrix adhesion (Ema) polypeptides. The invention provides nucleic acids encoding Group B streptococcal Ema polypeptides EmaA, EmaB, EmaC, EmaD and EmaE. The present invention provides isolated polypeptides comprising amino acid sequences of Group B streptococcal polypeptides EmaA, EmaB, EmaC, EmaD and EmaE, including analogs, variants, mutants, derivatives and fragments thereof. Ema homologous polypeptides from additional bacterial species, including S. pneumoniae, S. pyogenes, E. faecalis and C. diptheriae are also provided. Antibodies to the Ema polypeptides and immunogenic fragments thereof are also provided. The present invention relates to the identification and prevention of infections by virulent forms of streptococci. This invention provides pharmaceutical compositions, immunogenic compositions, vaccines, and diagnostic and therapeutic methods of use of the isolated polypeptides, antibodies thereto, and nucleic acids. Assays for compounds which modulate the polypeptides of the present invention for use in therapy are also provided.
Owner:UNIV OF UTAH RES FOUND +1
Immunogenic composition
InactiveUS20140193451A1High activityBacterial antigen ingredientsDepsipeptidesImmunogenicityVirology
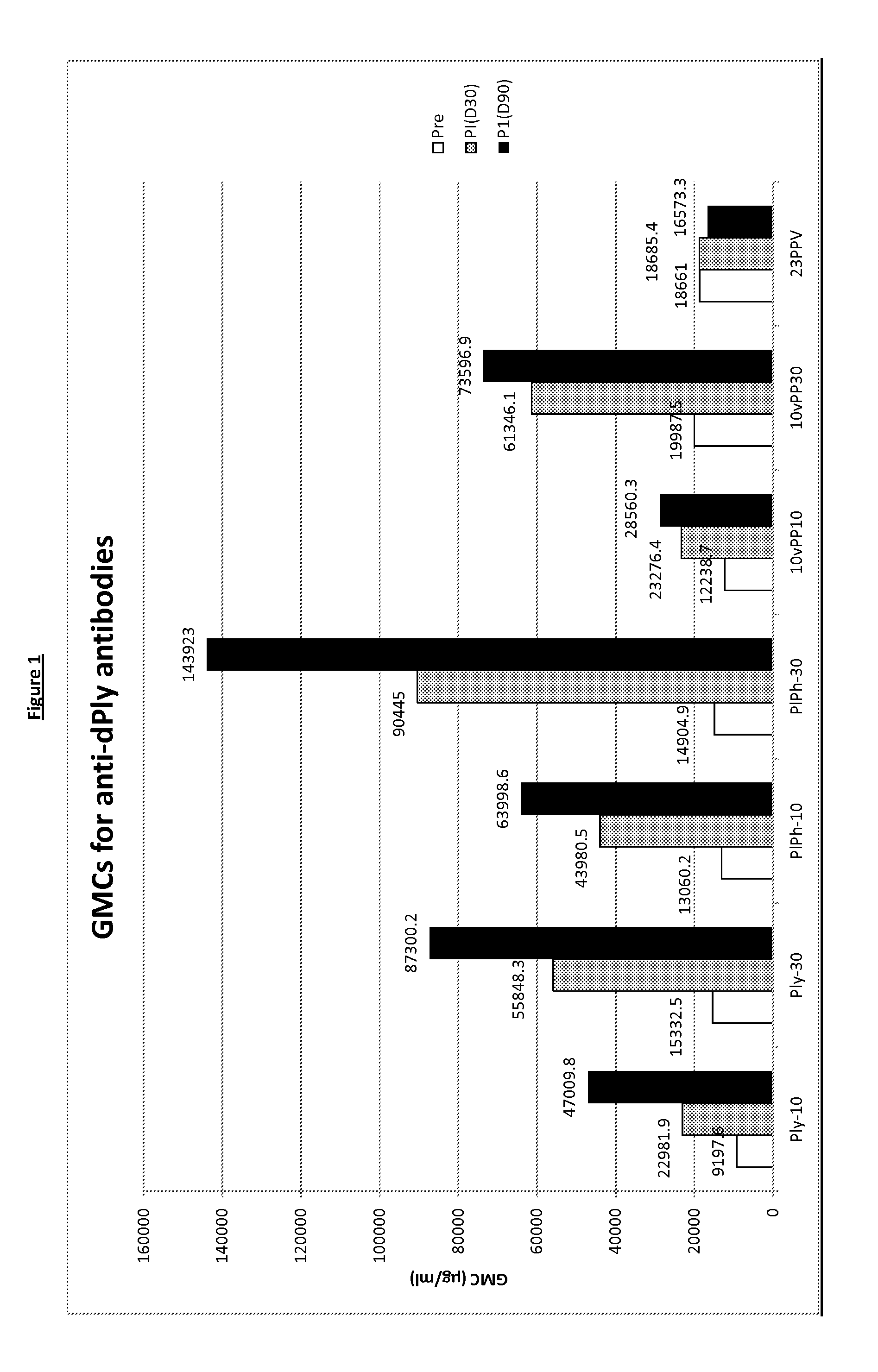
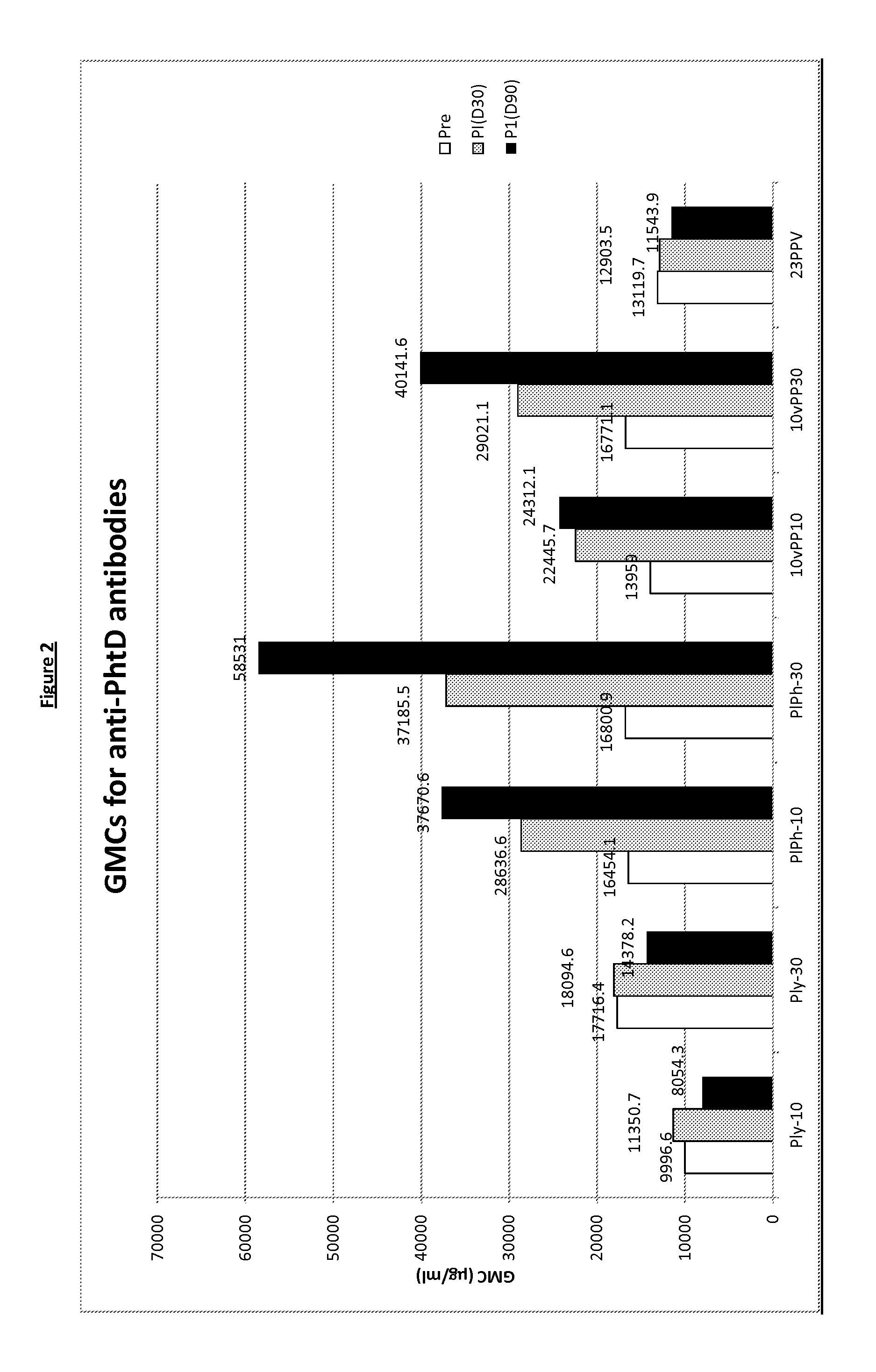
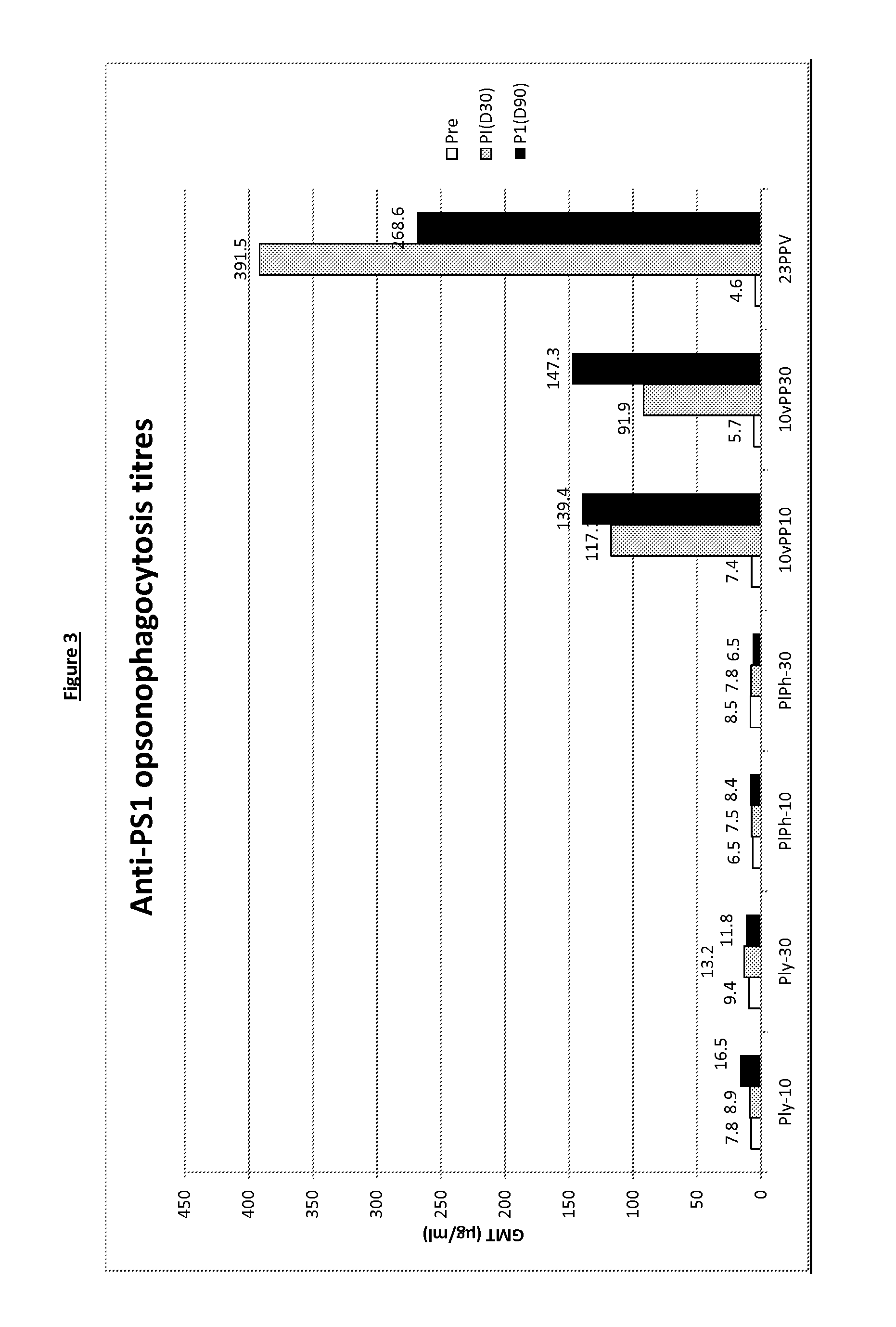
The present invention relates to immunogenic compositions comprising 26 μg-45 μg of pneumolysin and / or PhtD, vaccines comprising the immunogenic compositions and their use in medicine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
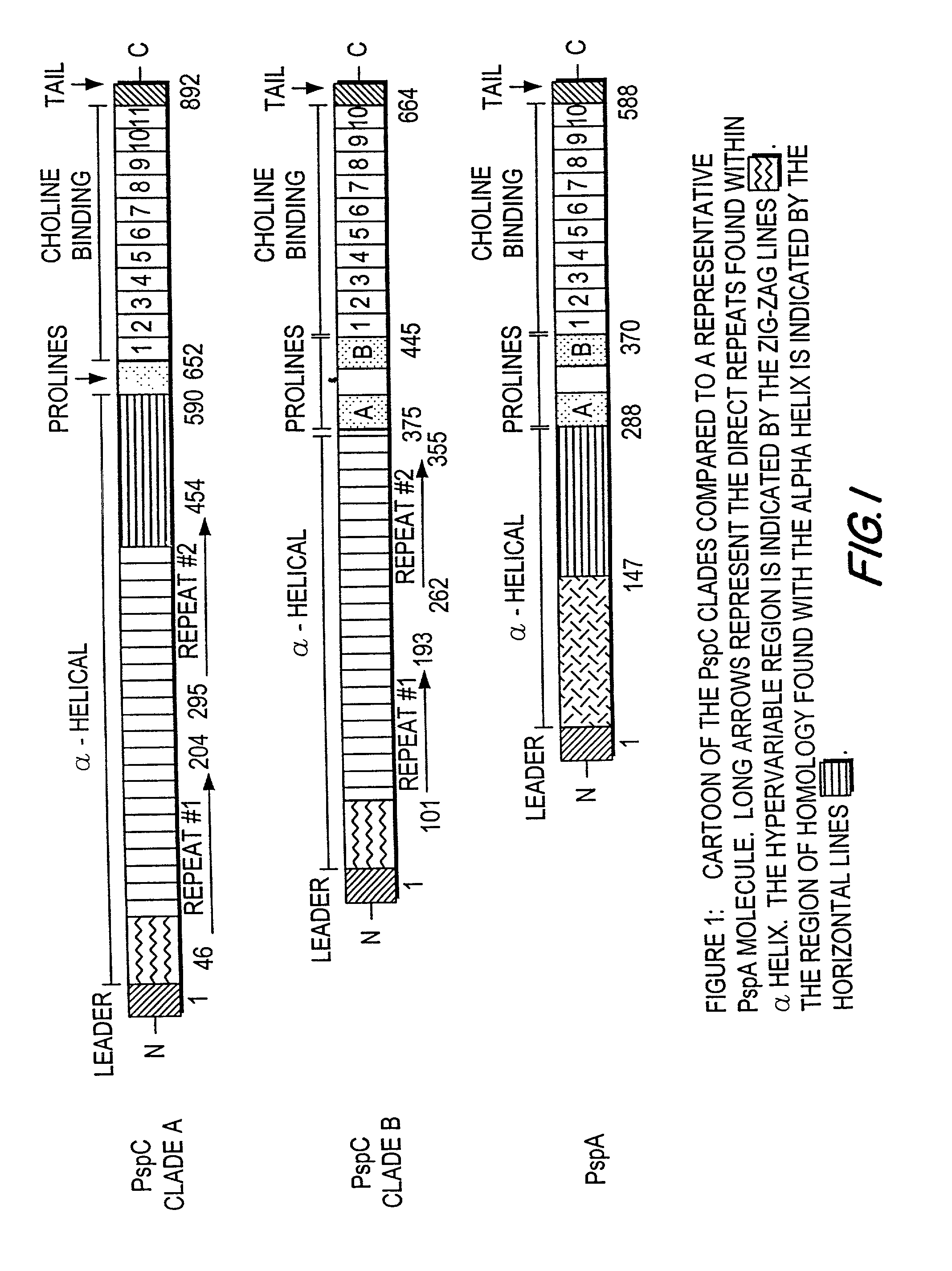
Pneumococcal surface protein C (PspC), epitopic regions and strain selection thereof, and uses therefor
InactiveUS20010016200A1Improving immunogenicityBacterial antigen ingredientsProtozoa antigen ingredientsEukaryotic plasmidsIn vivo
Disclosed and claimed are: epitopic regions of Pneumococcal Surface Protein C or "PspC", different clades of PspC, isolated and / or purified nucleic acid molecules such as DNA encoding a fragment or portion of PspC such as an epitopic region of PspC or at least one epitope of PspC, uses for such nucleic acid molecules, e.g., to detect the presence of PspC or of S. pneumoniae by detecting a nucleic acid molecule therefor in a sample such as by amplification and / or a polymerase chain reaction, vectors or plasmids which contain and / or express such nucleic acid molecles, e.g., in vitro or in vivo, immunological, immunogenic or vaccine compositions including at least one PspC and / or a portion thereof (such as at least one epitopic region of at least one PspC and / or at least one polypeptide encoding at least one epitope of at least one PspC), either alone or in further combination with at least one second pneumococcal antigen, such as at least one different PspC and / or a fragment thereof and / or at least one PspA and / or at least one epitopic region of at least one PspA and / or at least one polypeptide including at least one epitope of PspA. PspC or a fragment thereof, and thus a composition including PspC or a fragment thereof, can be administered by the same routes, and in approximately the same amounts, as PspA. Thus, the invention further provides methods for administering PspC or a fragment thereof, as well as uses of PspC or a fragment thereof to formulate such compositions.
Owner:UAB RES FOUND
Immunogenic compositions for use in pneumococcal vaccines
ActiveUS20190000952A1Raise the ratioIncrease OPA titerBacterial antigen ingredientsAntiinfectivesCoccidiaSalmonella serotype typhi
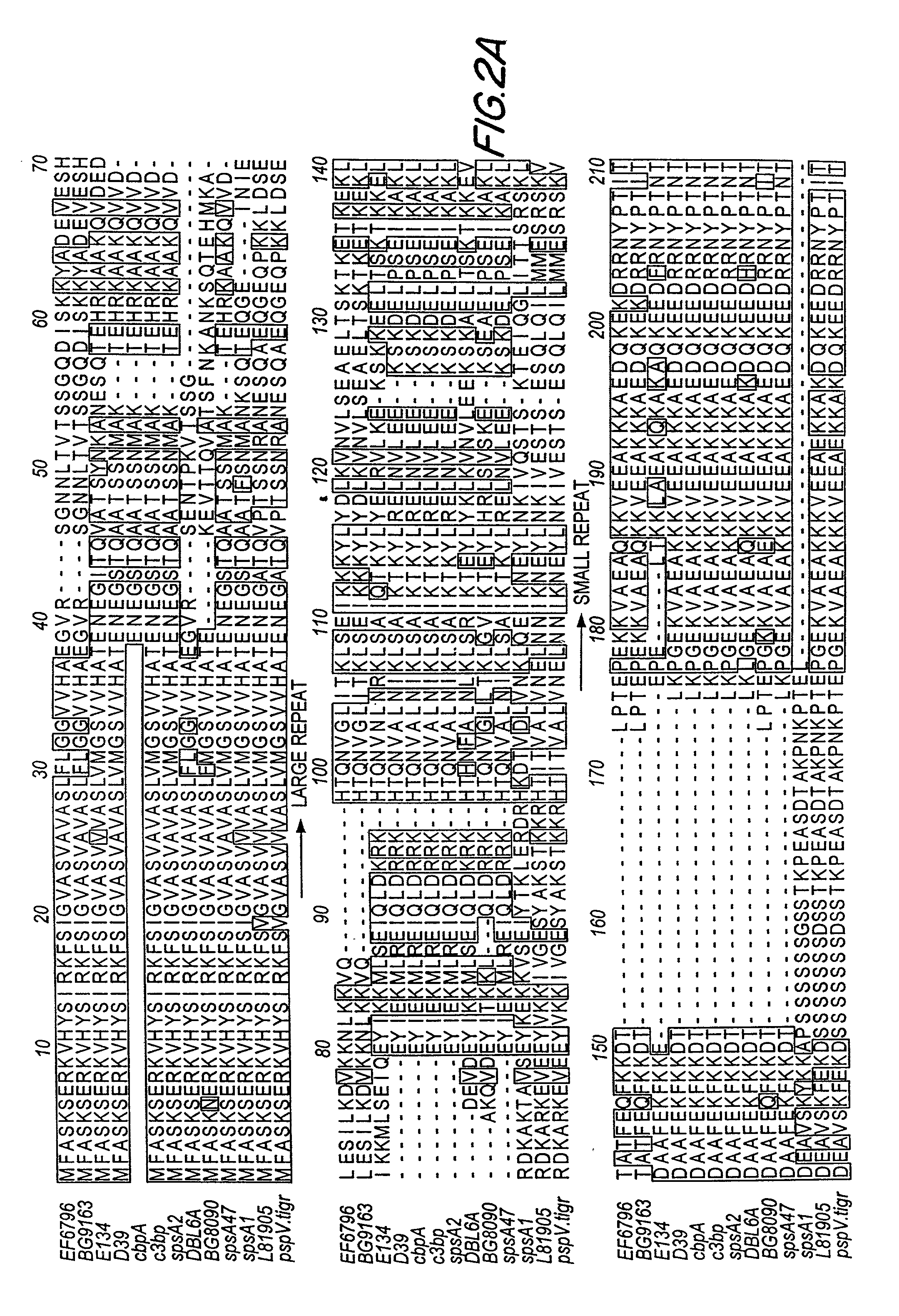
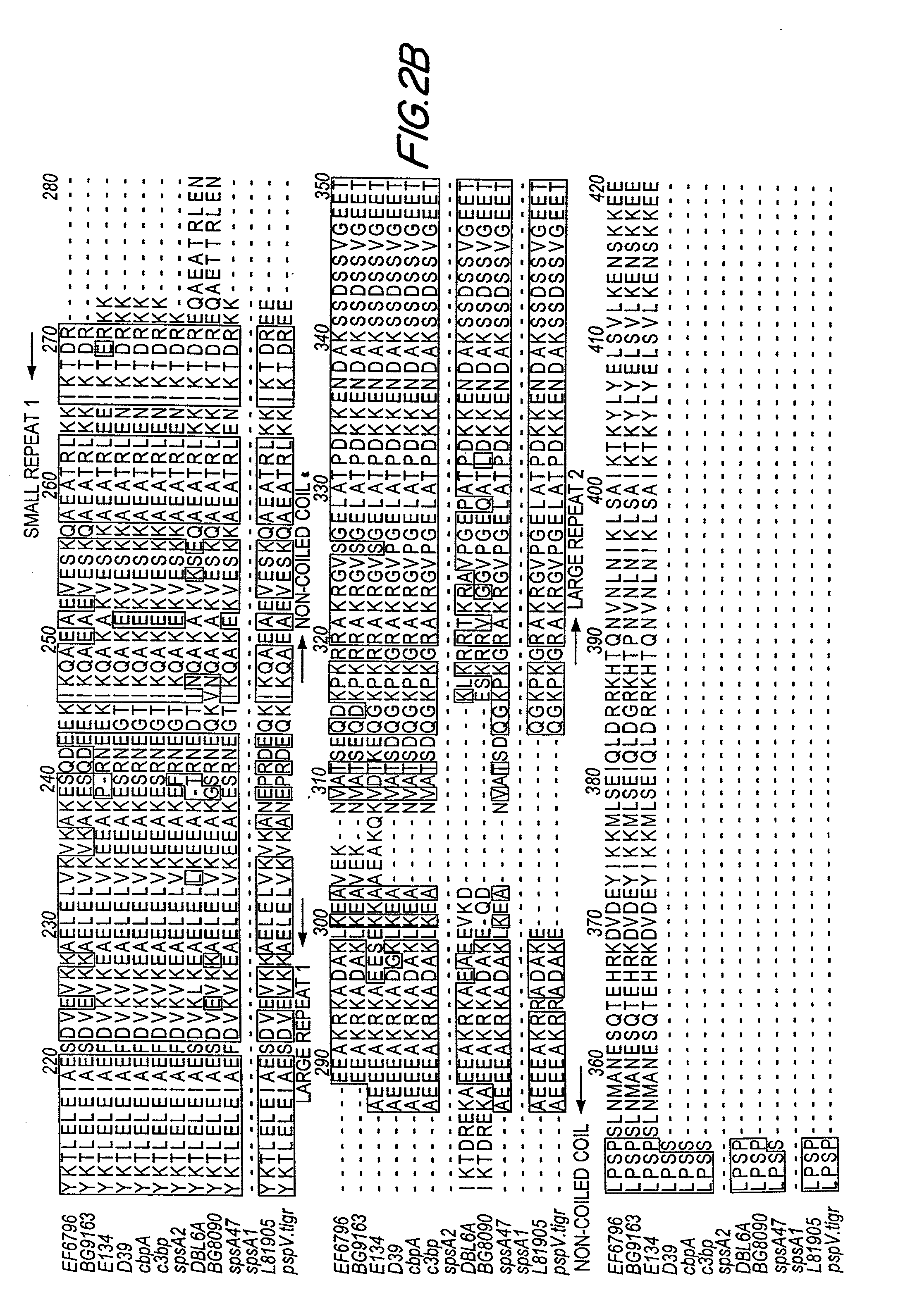
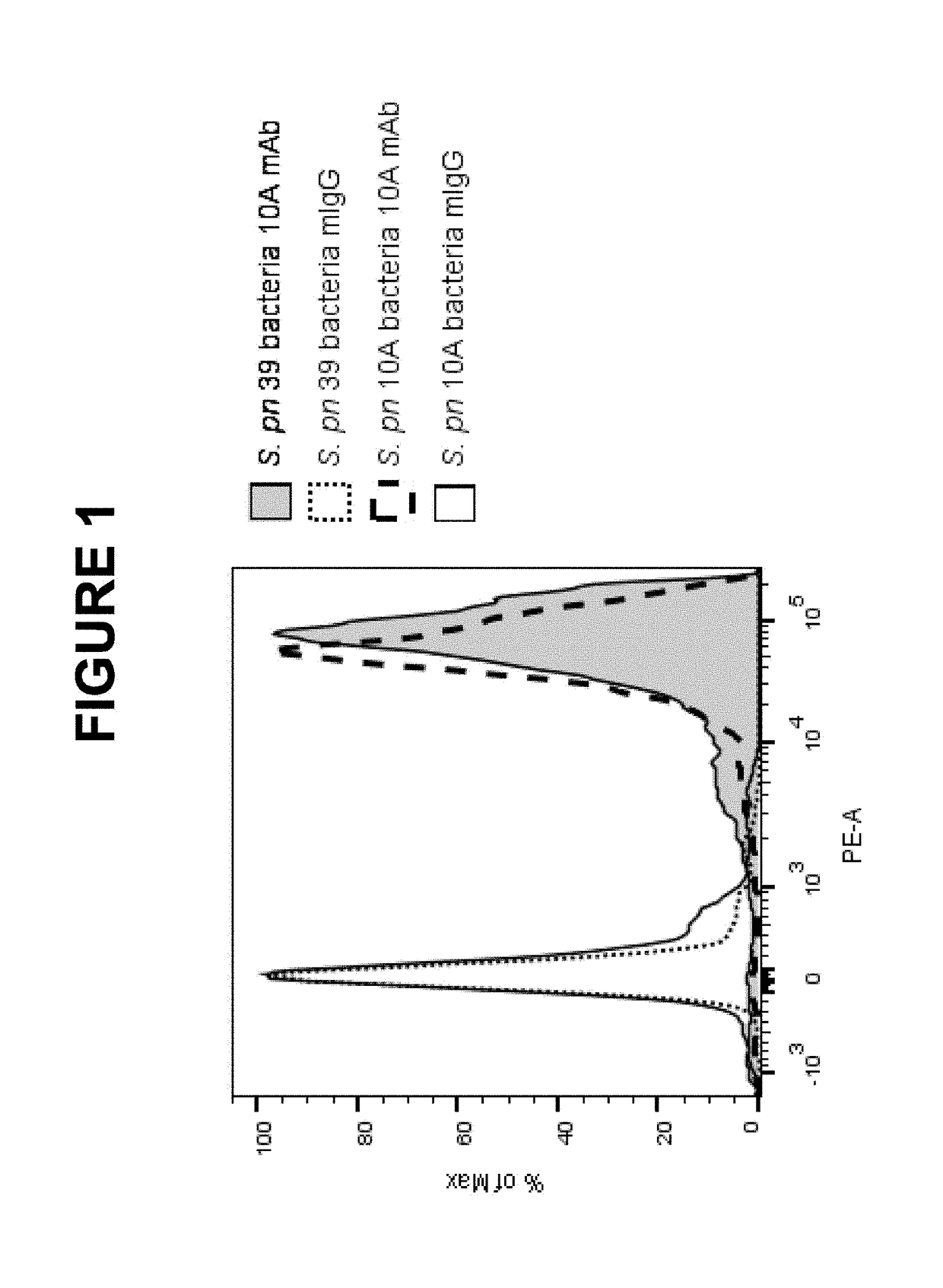
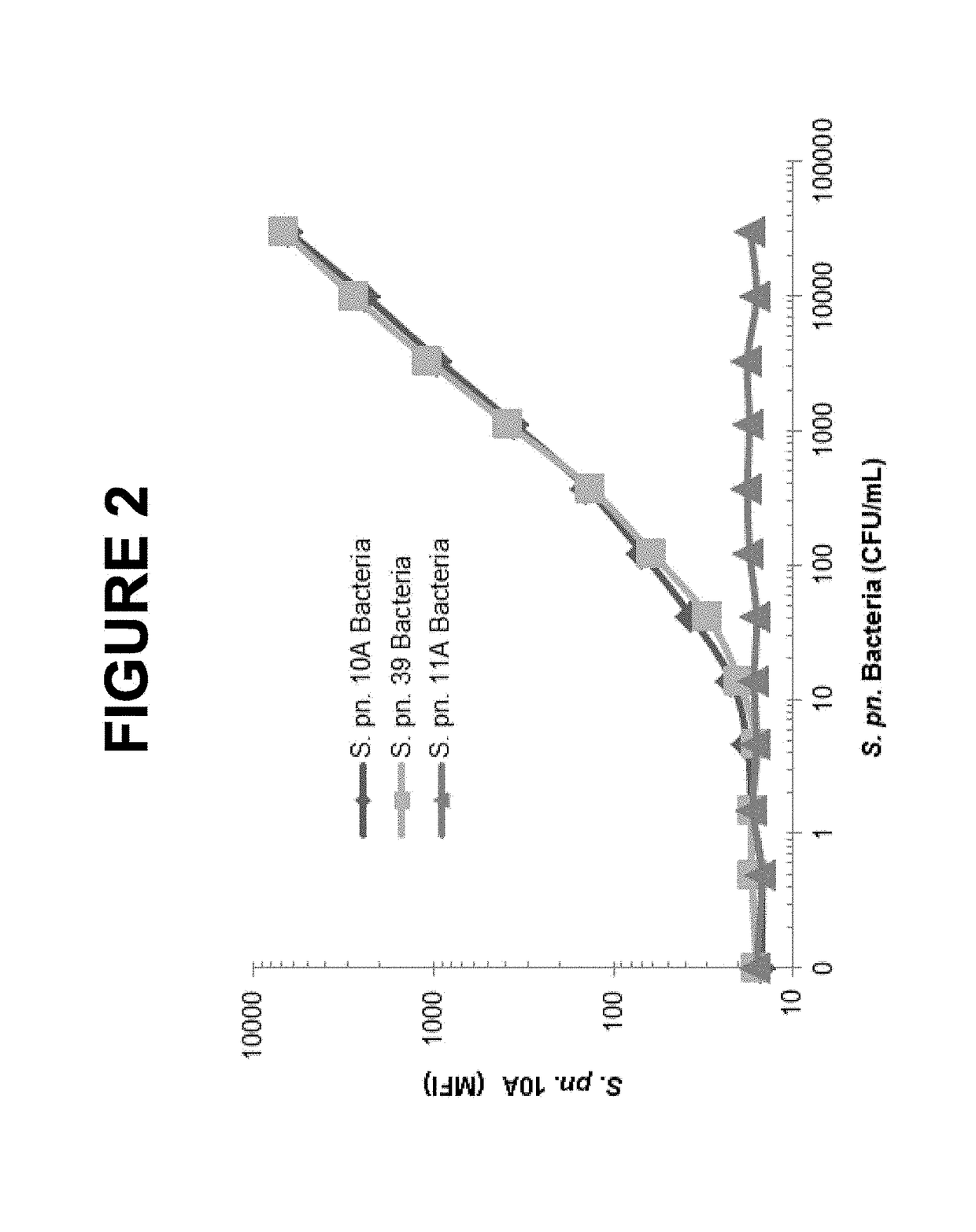
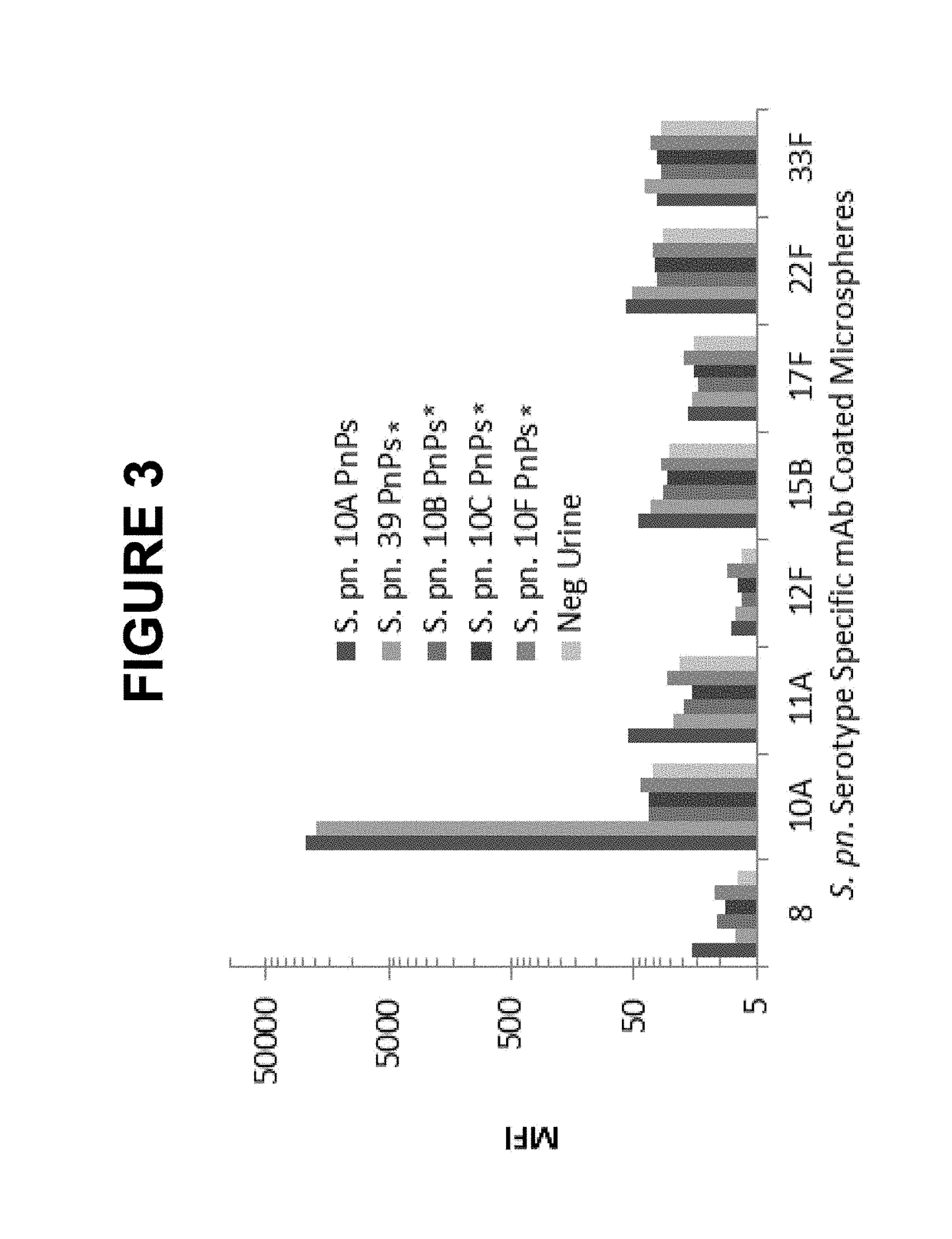
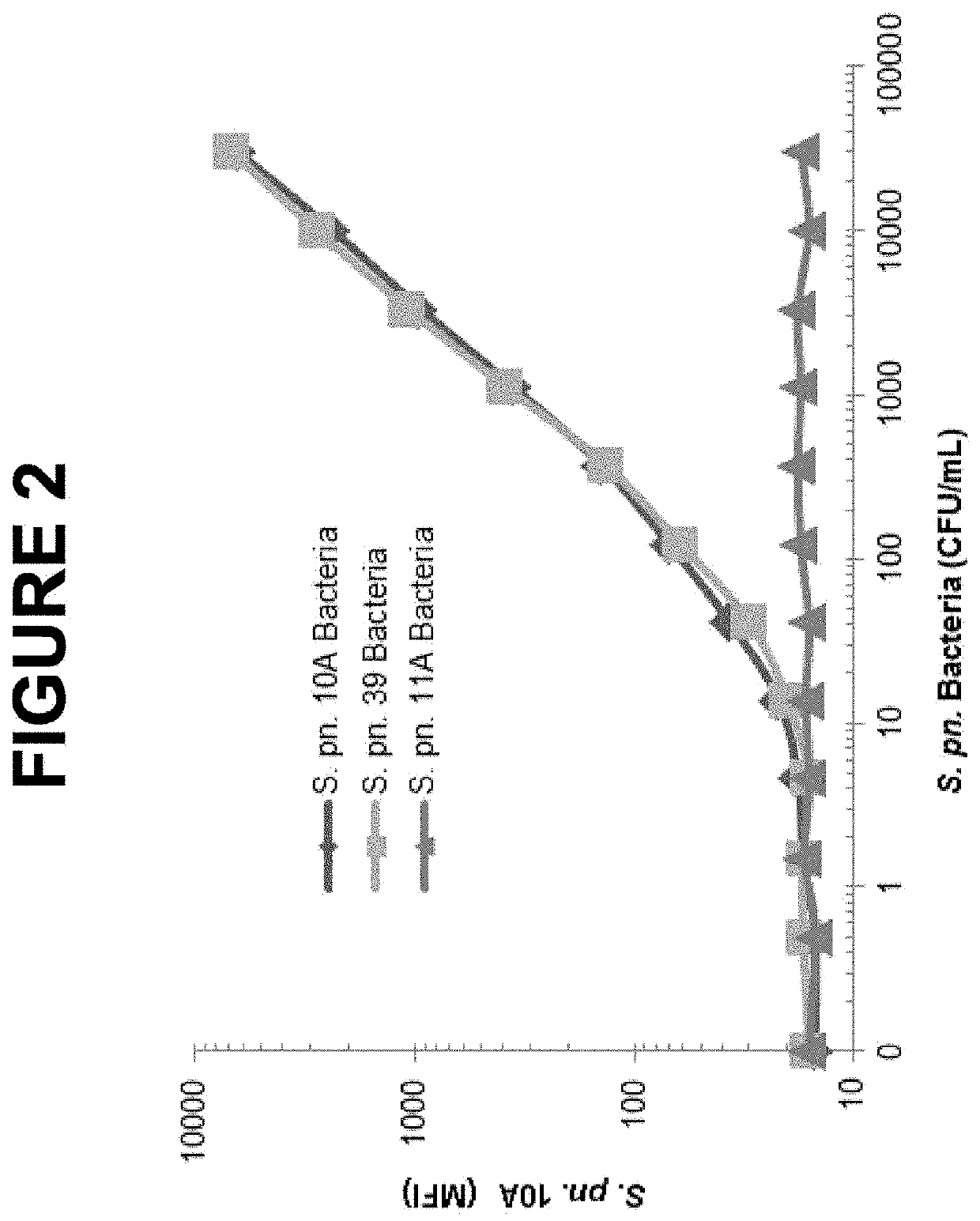
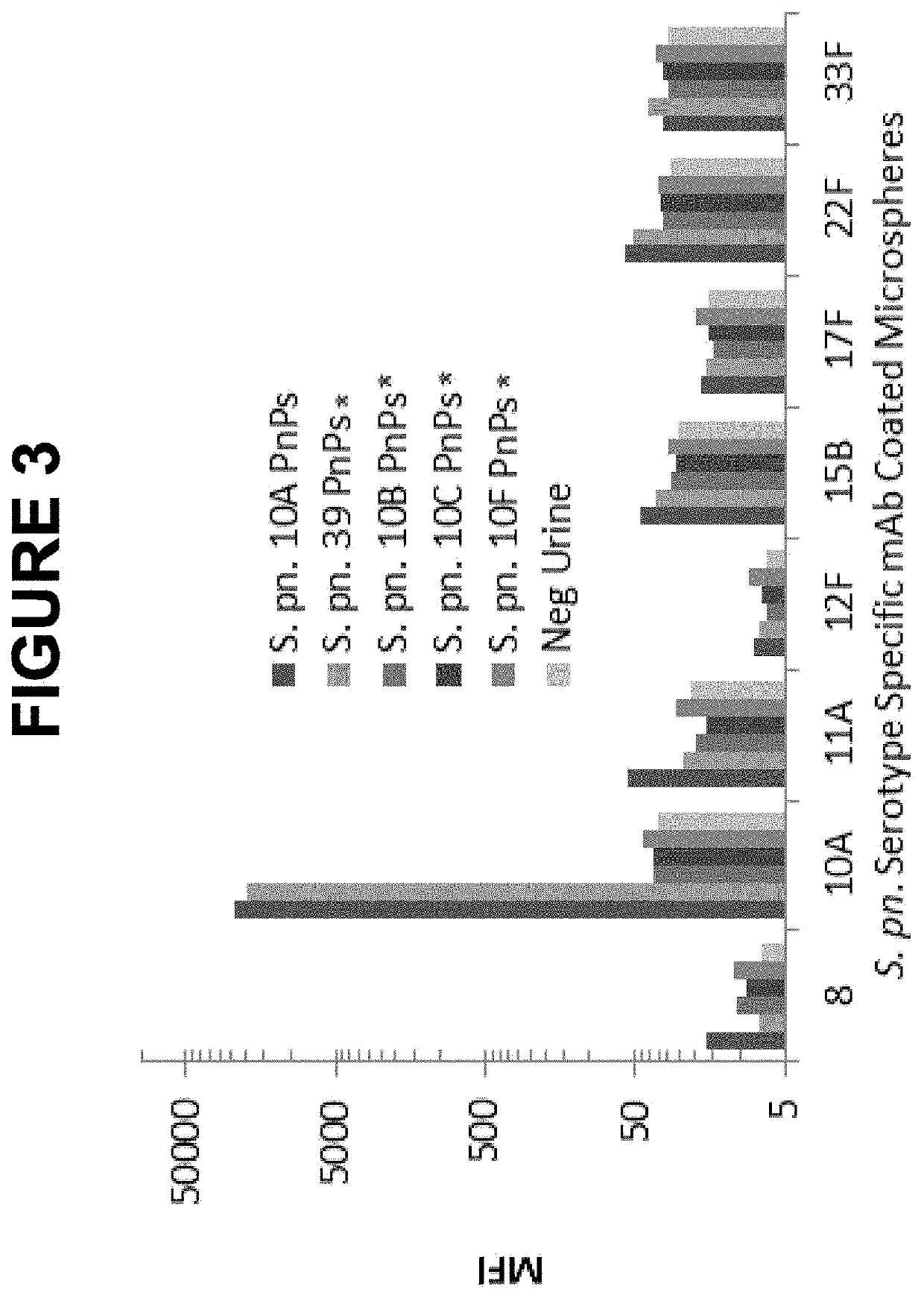
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Mucosal combination vaccines for bacterial meningitis
InactiveUS20060147466A1Improving immunogenicityLarge capacityAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeHaemophilus
A composition for mucosal delivery, comprising two or more of the following: (a) an antigen which induces an immune response against Haemophilus influenzae; (b) an antigen which induces an immune response against Neisseria meningitidis; and (c) an antigen which induces an immune response against Streptococcus pneumoniae. The combination allows a single dose for immunising against three separate causes of a common disease, namely bacterial meningitis.
Owner:CHIRON CORP
Immunogenic compositions for use in pneumococcal vaccines
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20200306357A1Bacterial antigen ingredientsPharmaceutical non-active ingredientsPrevnar 13Glycoconjugate
Owner:PFIZER INC
Multivalent Pneumococcal Glycoconjugate Vaccines Containing Emerging Serotype 24F
PendingUS20220040283A1Antibacterial agentsBacterial antigen ingredientsPneumococcal serotypesStreptococcus pneumoniae capsular polysaccharide
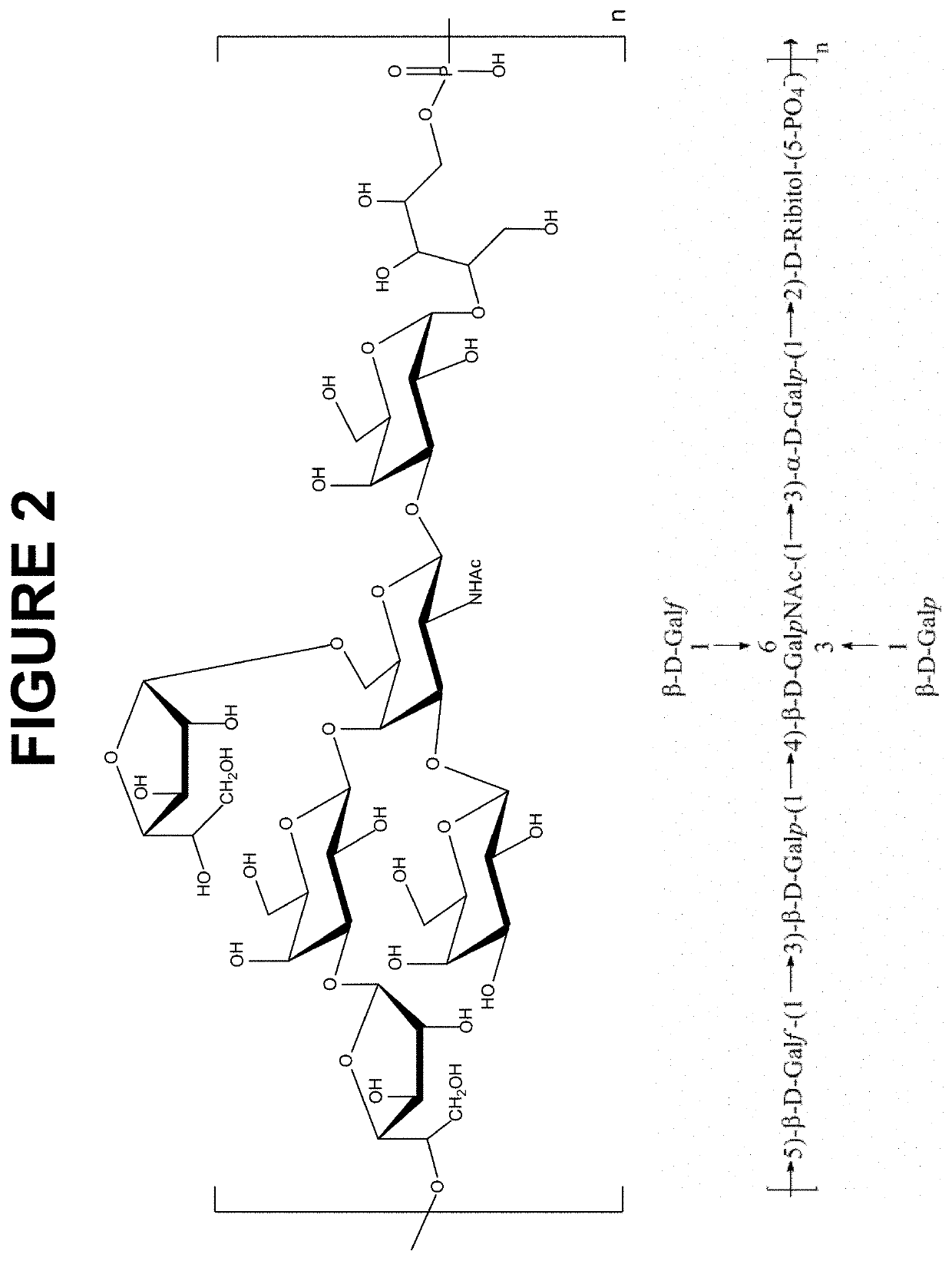
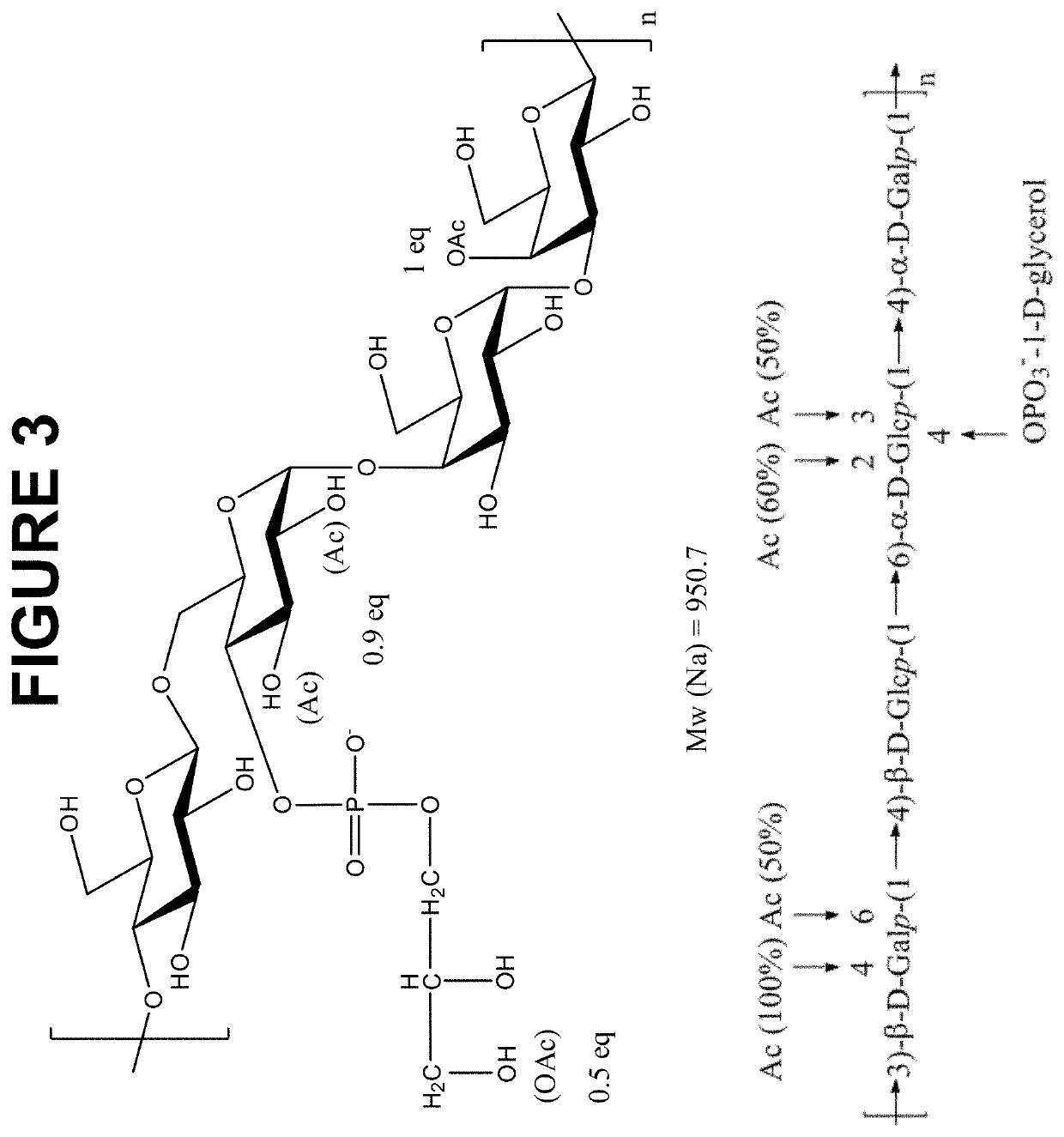
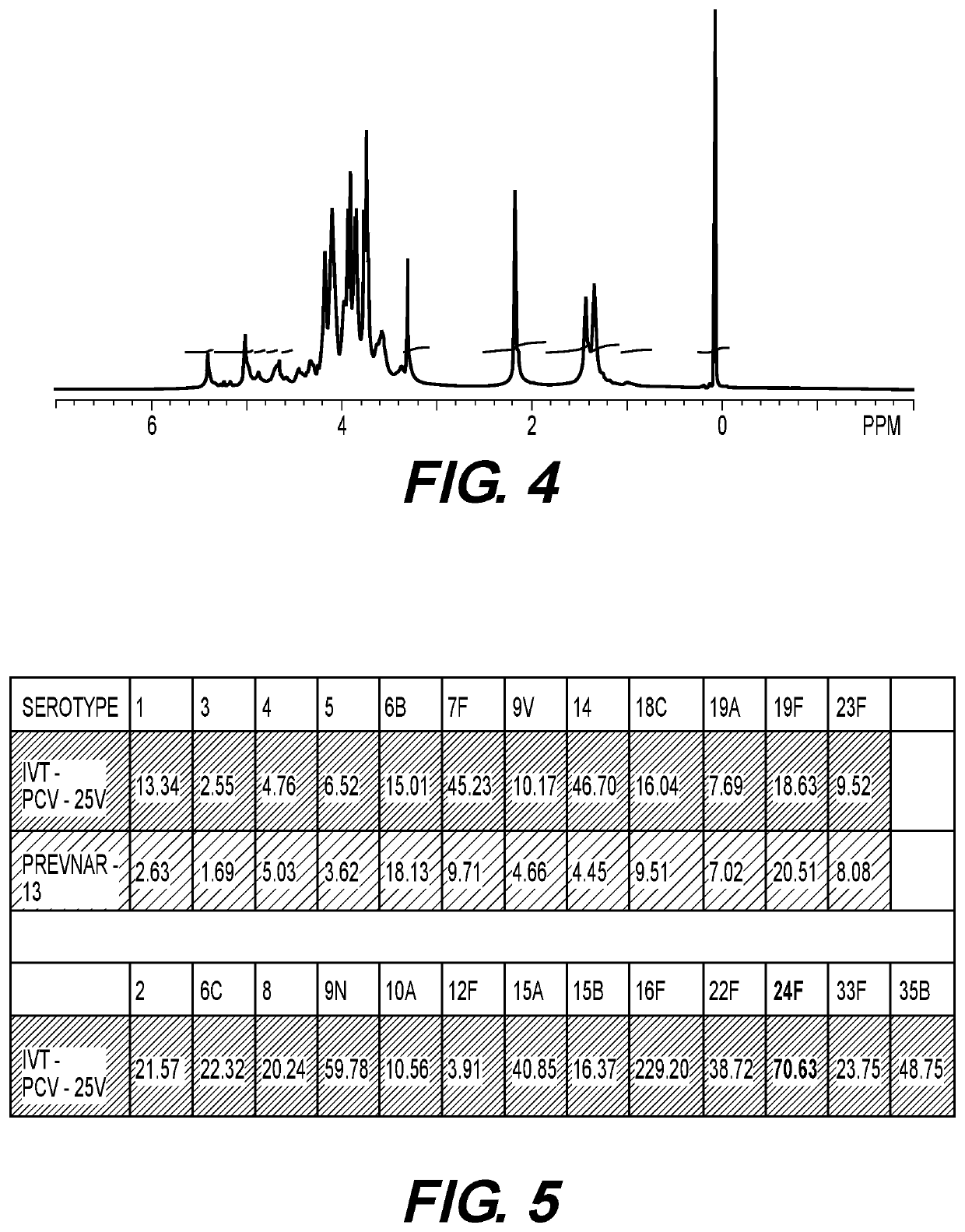
The invention is directed to multivalent pneumococcal glycoconjugate compositions containing a newly emerging serotype of S. pneumonia, polysaccharide 24F, and their manufacture and use. The pneumococcal serotype 24F contains a polysaccharide repeating unit structure. The multivalent immunogenic composition comprises at least 25 S. pneumonia capsular polysaccharides selected from the serotypes 1, 2, 3, 4, 5, 6B, 6C, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15A, 15B, 15C, 16F, 17F, 18C, 19A, 19F, 20, 22F, 23B, 23F, 24F, 33F and 35B of S. pneumoniae preferably conjugated to carrier protein either directly or through a linker and a pharmaceutically acceptable carrier and / or adjuvant. The disclosure provides the capsular polysaccharide structure of serotype 24F to understand the polysaccharide before conjugation with carrier protein.
Owner:INVENTPRISE INC
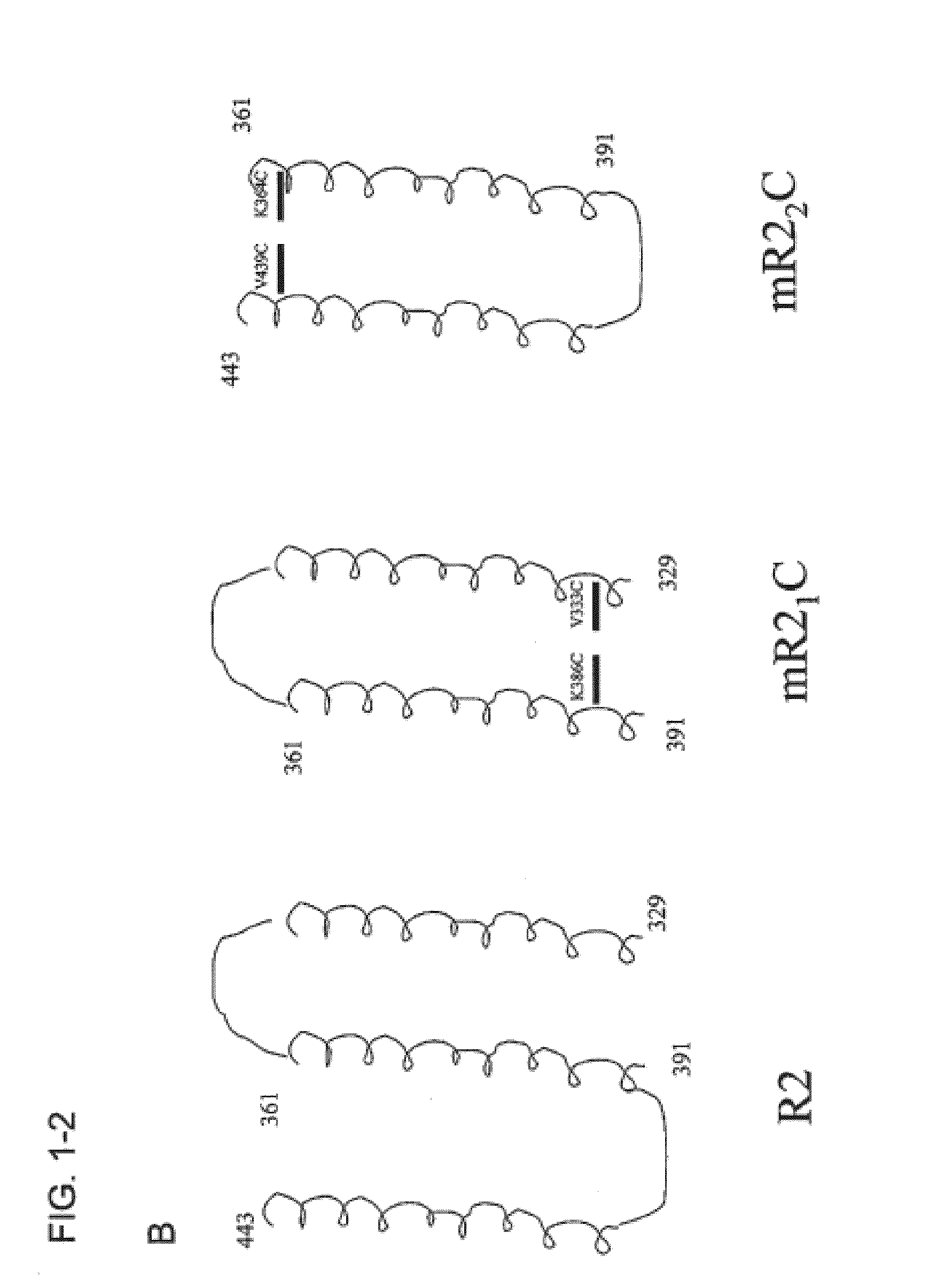
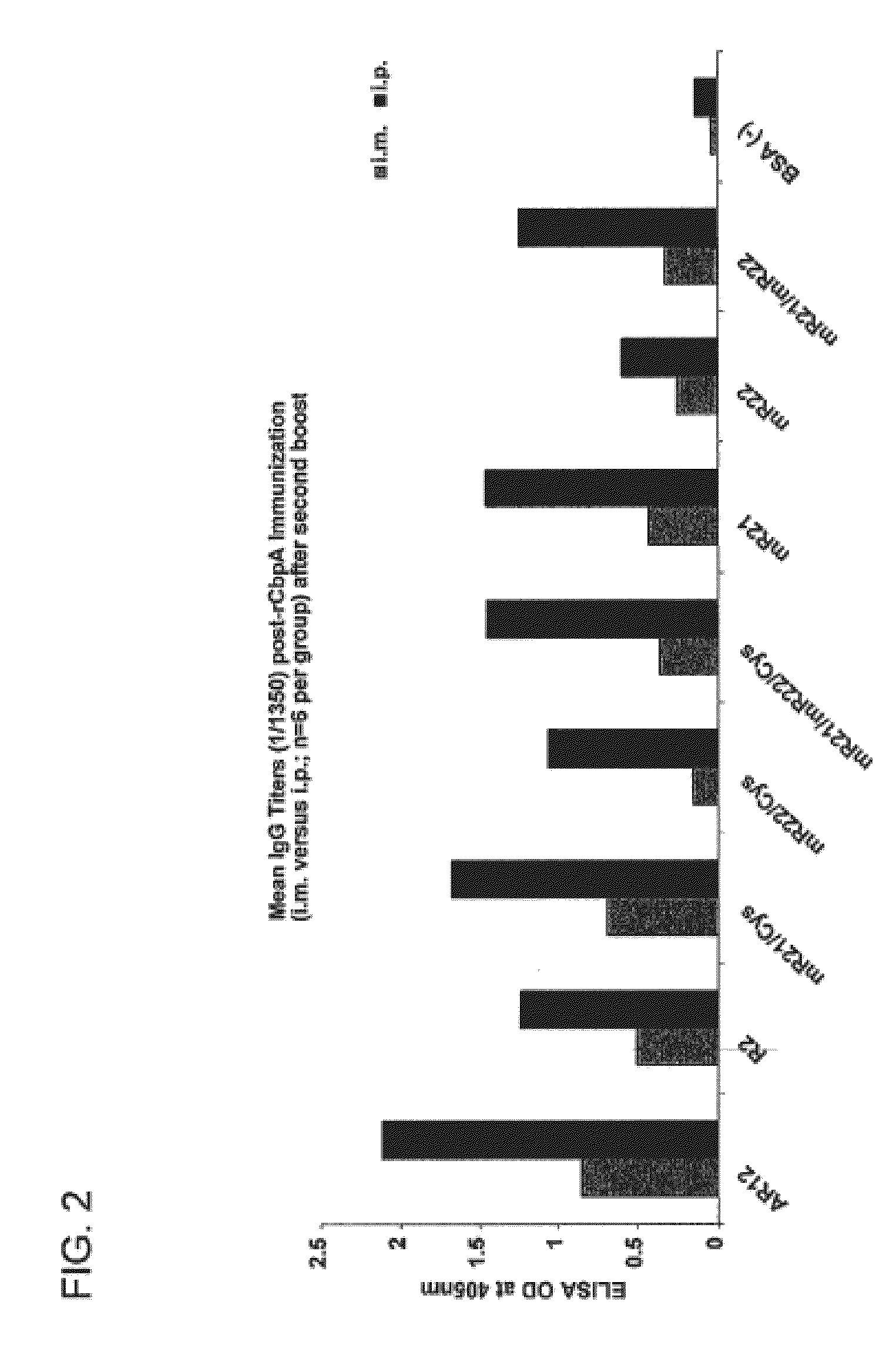
Synthetic Streptococcus pneumoniae vaccine
Compositions and methods for preventing and treating pneumococcal infections are provided. Compositions include novel polypeptides comprising an amino acid sequence corresponding to the R2i or R22 domain of CbpA or a consensus sequence of one of these domains, and variants and fragments thereof, wherein the polypeptide is stabilized in a desired conformation, particularly a loop conformation. The polypeptides of the invention may be engineered to comprise a first and a second cysteine residue, thereby resulting in the formation of a disulfide bond that stabilizes the polypeptide in the desired conformation. Alternatively, a polypeptide of the invention may be modified to create a synthetic linkage between a first and second amino acid residue present within the polypeptide, wherein the synthetic linkage stabilizes the polypeptide in the desired conformation. The polypeptides of the invention may further comprise an amino acid sequence for a T cell epitope. Compositions further include isolated nucleic acid molecules that encode the polypeptides of the invention, immunogenic compositions and vaccines comprising the disclosed polypeptides, and antibodies specific for these polypeptides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Multivalent pneumococcal conjugate vaccine
ActiveUS11147863B2Antibacterial agentsBacterial antigen ingredientsCarrier proteinPharmaceutical medicine
The present invention relates to a multivalent Pneumococcal conjugate vaccine (PCV) composition comprising: 1) at least 12 capsular polysaccharides selected from serotypes 1, 3, 4, 5, 6B, 7F, 9N, 9V, 15B, 14, 18C, 19A, 19F, 22F, 23F and 33F of S. pneumoniae activated with CDAP and conjugated to carrier protein selected from CRM197, pneumococcal surface protein A (PspA), pneumococcal adhesin protein (PsaA) or combination thereof and 2) a pharmaceutically acceptable carrier, wherein the composition does not contain capsular polysaccharide from serotype 6A.
Owner:BIOLOGICAL E LTD
An immunogenic serotype 35b pneumococcal polysaccharide-protein conjugate and conjugation process for making the same
PendingUS20220233674A1Antibacterial agentsBacterial antigen ingredientsCarrier proteinStreptococcus halichoeri
The present invention provides a process improvement related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae (S. pneumoniae) serotype 35B to a carrier protein. The serotype 35B polysaccharide-protein conjugate, prepared by the disclosed process, is, among other things, more immunogenic than similar conjugates made by prior art methods. S. pneumoniae serotype 35B polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccine compositions.
Owner:MERCK SHARP & DOHME LLC
Pneumonia pathogenic bacteria rapid identification gene chip
ActiveCN107287311BNucleotide librariesMicrobiological testing/measurementPseudomonasAerobacter cloacae
The invention discloses a rapid-recognition gene chip for pathogenic bacteria of pneumonia. The gene chip can detect 15 pathogenic bacteria including streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae, and clinically common and difficult-to-culture pathogenic bacteria are contained. In a preparation process of the gene chip, design, screening and verification of probes are performed by adopting 16S rDNA and a specific gene sequence corresponding to each of the pathogenic bacteria, and types of the bacteria in a to-be-detected sample are identified from levels of genus and species simultaneously and respectively. The gene chip has the advantages that the defect that clinical detection of the pathogenic bacteria of pneumonia is not timely and comprehensive at present is overcome, and one novel detection way is provided for early diagnosis and early treatment of patients with pneumonia.
Owner:GENERAL HOSPITAL OF PLA +1
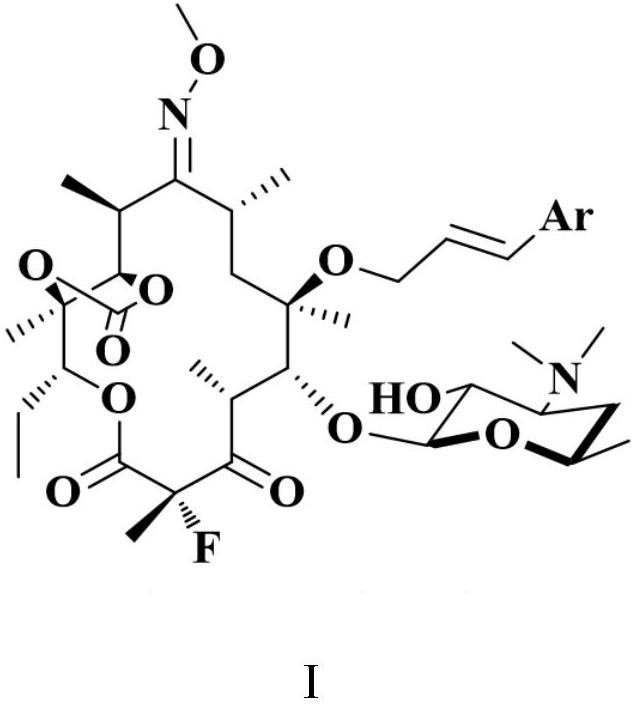
A kind of erythromycin derivative and preparation method thereof
ActiveCN109942653BEffective treatmentAdapt to industrial productionAntibacterial agentsOrganic active ingredientsOrganic acid formationInfluenza a
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Application of L-rhamnose antibody in preparation of medicine for preventing and/or treating drug-resistant bacterial infection
PendingCN113648409AStrong anti-kill abilityStrong broad spectrumAntibacterial agentsAerosol deliveryEscherichia coliNatural antibody
The invention relates to application of an L-rhamnose antibody in preparation of a drug for preventing and / or treating drug-resistant bacterial infection. The L-rhamnose antibody is used for specifically recognizing L-rhamnose, and the L-rhamnose antibody is a human source L-rhamnose natural antibody or a mouse source L-rhamnose monoclonal antibody; the drug-resistant bacteria particularly belong to drug-resistant bacteria of which the genome contains an L-rhamnose synthesis route. According to the application disclosed by the invention, L-rhamnose synthetic pathways exist in genomes of a considerable part of drug-resistant bacteria, so that L-rhamnose exists in polysaccharide structures on the surfaces of many drug-resistant bacteria, and L-rhamnose antibodies can recognize and be combined with the L-rhamnose structures on the surfaces of the drug-resistant bacteria; further, the compound has extremely strong inhibiting and killing capability on various drug-resistant bacteria including pseudomonas aeruginosa, escherichia coli and streptococcus pneumoniae, and has the advantages of broad spectrum and good killing effect.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Strain culture medicine favorable for preventing bacterial contamination
InactiveCN107760635AIncrease proliferation rateLess prone to bacterial contaminationBacteriaMicroorganism based processesCodonopsisCorn syrup
The invention discloses a strain culture medicine favorable for preventing bacterial contamination. The culture medium comprises the following raw materials in parts by weight: 15 to 45 parts of jerusalem artichoke, 10 to 30 parts of codonopsis pilosula, 10 to 30 parts of medlar leaf, 20 to 80 parts of peptone, 15 to 55 parts of corn pulp, 10 to 35 parts of bran, 0.25 to 2.0 g / L of MgSO4, 2.0 to 12.0 g / L of NaCl, 0.8 to 3.0 g / L of K2HPO4, 0.1 to 0.3 g / L of ZnSO4, 0.01 to 0.08 g / L of FeSO4, 0.2 to 1.2 g / L of nicotinic acid, 3.5 to 8.5 g / L of adenine, 0.8 to 2.0 g / L of cysteine, 15 to 20 parts of agar and 1000 parts of water. The culture medium promotes obvious increment of proliferation rate of streptococcus pneumonia and is not liable to bacterial contamination.
Owner:CHENGDU OLYMVAX BIOPHARM
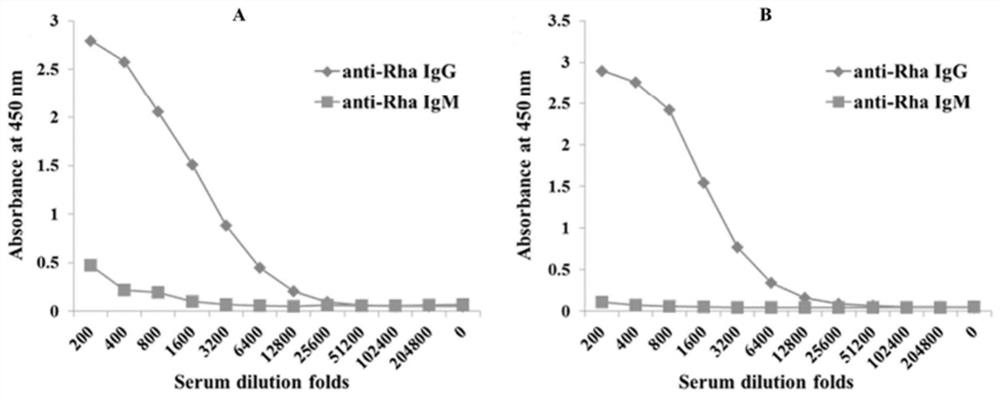
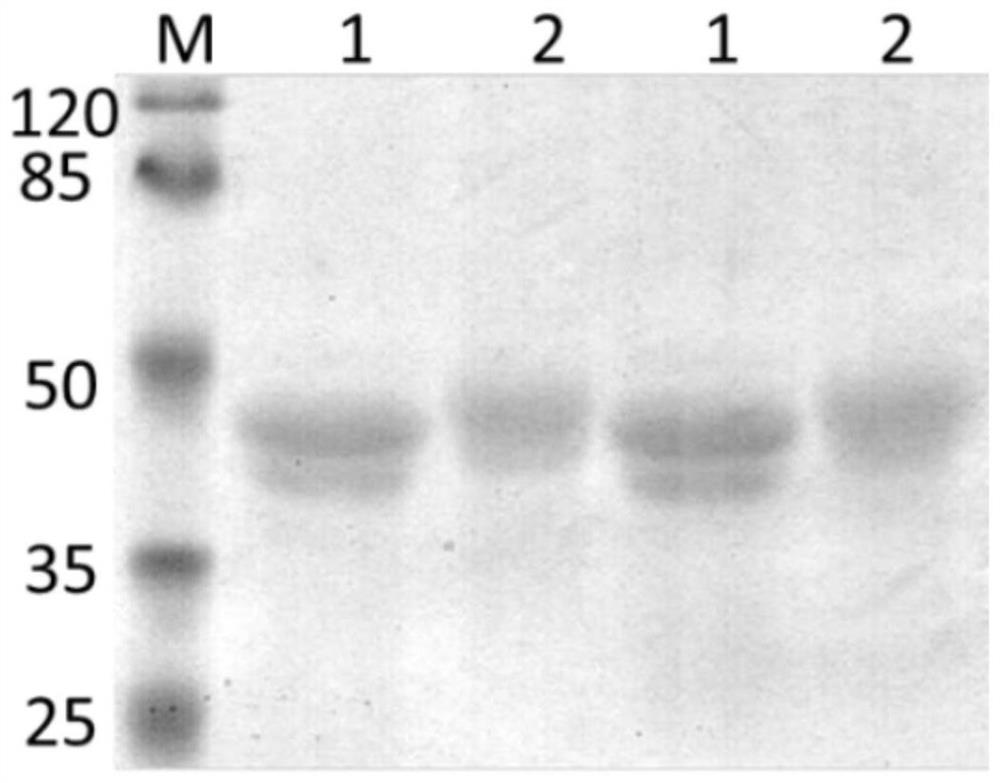
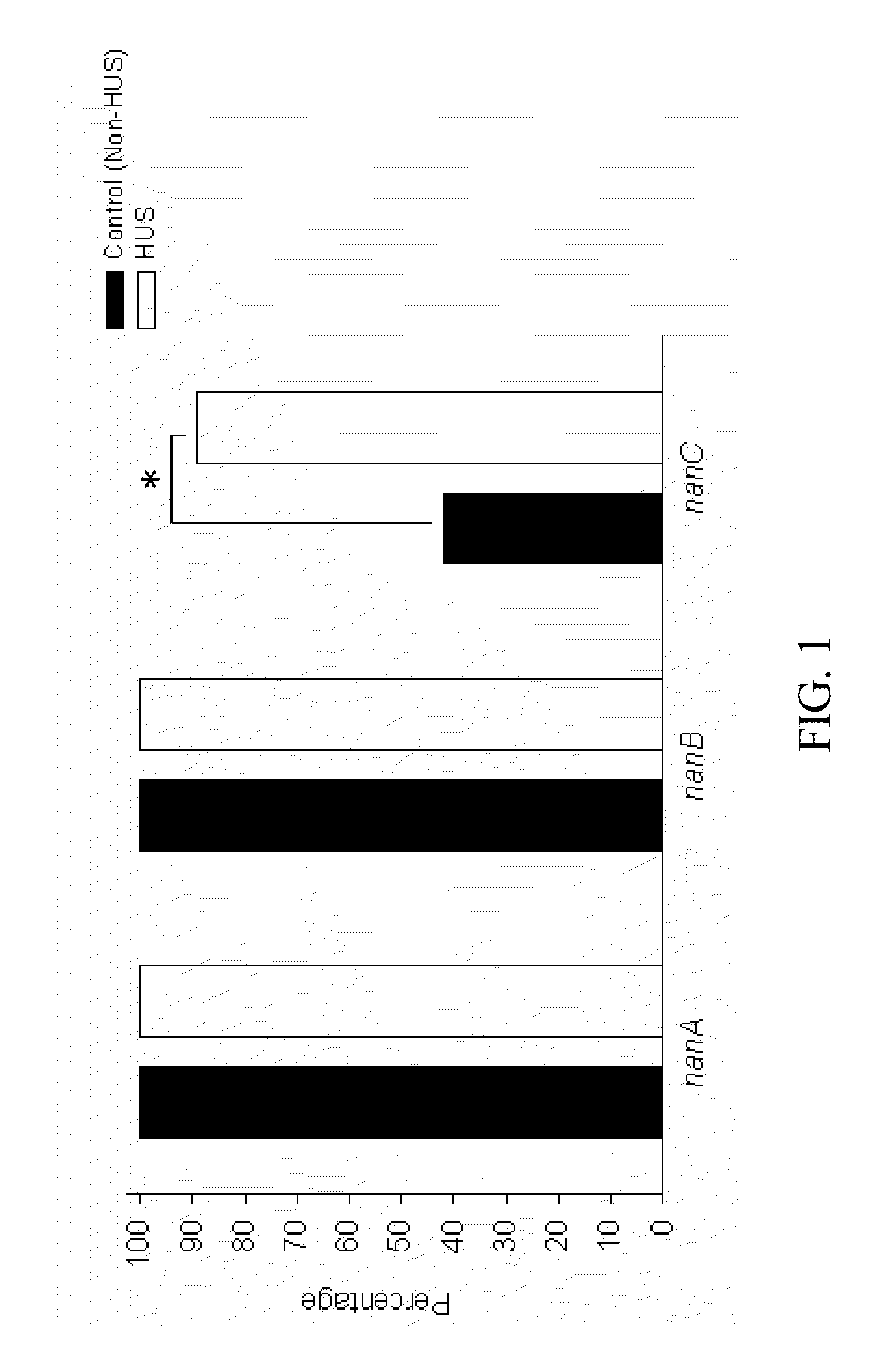
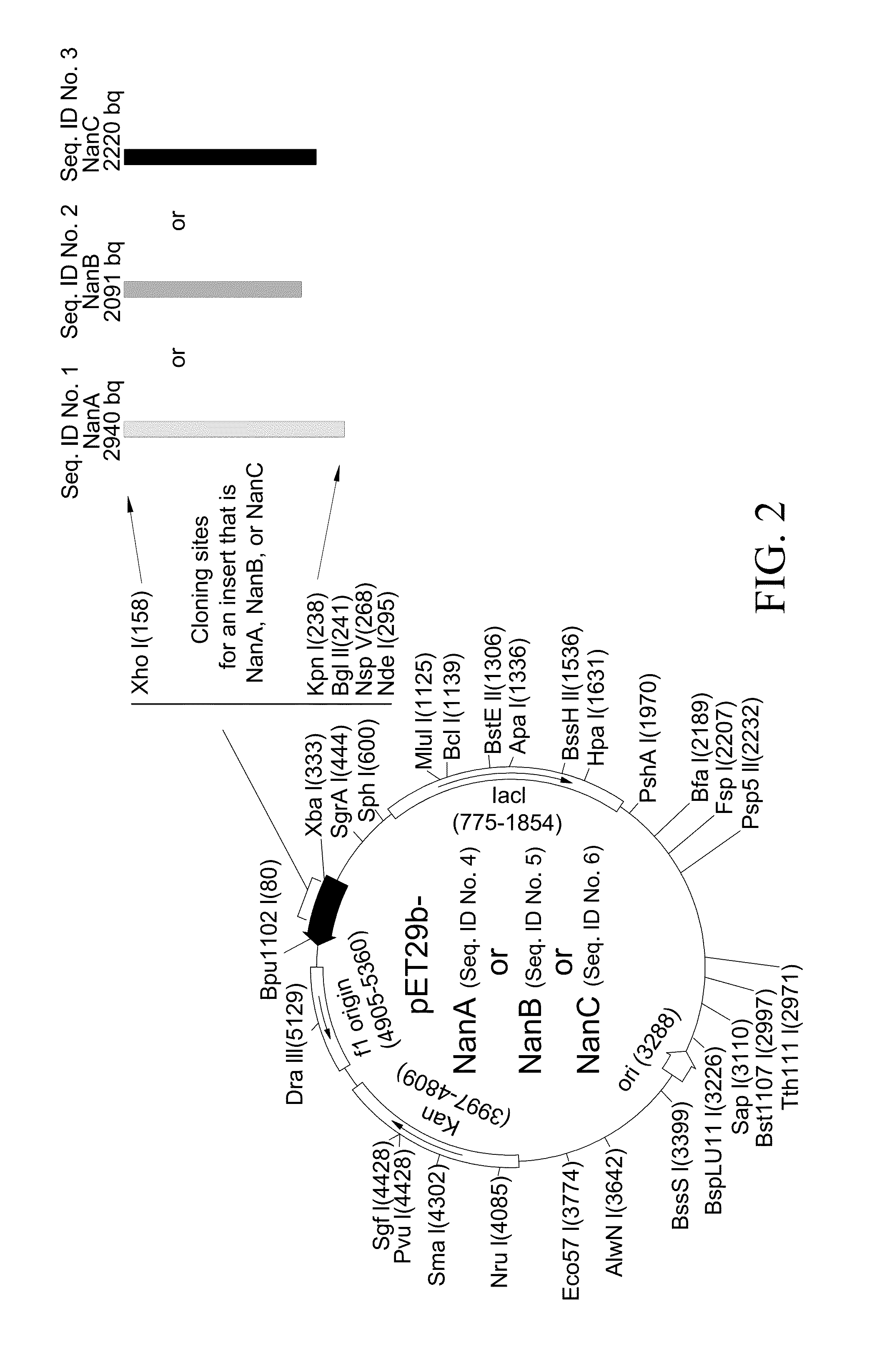
Method of diagnosing and preventing pneumococcal diseases using pneumococcal neuraminidases
InactiveUS20140242591A1Detect presenceMicrobiological testing/measurementAntibody medical ingredientsAdjuvantNeuraminidase
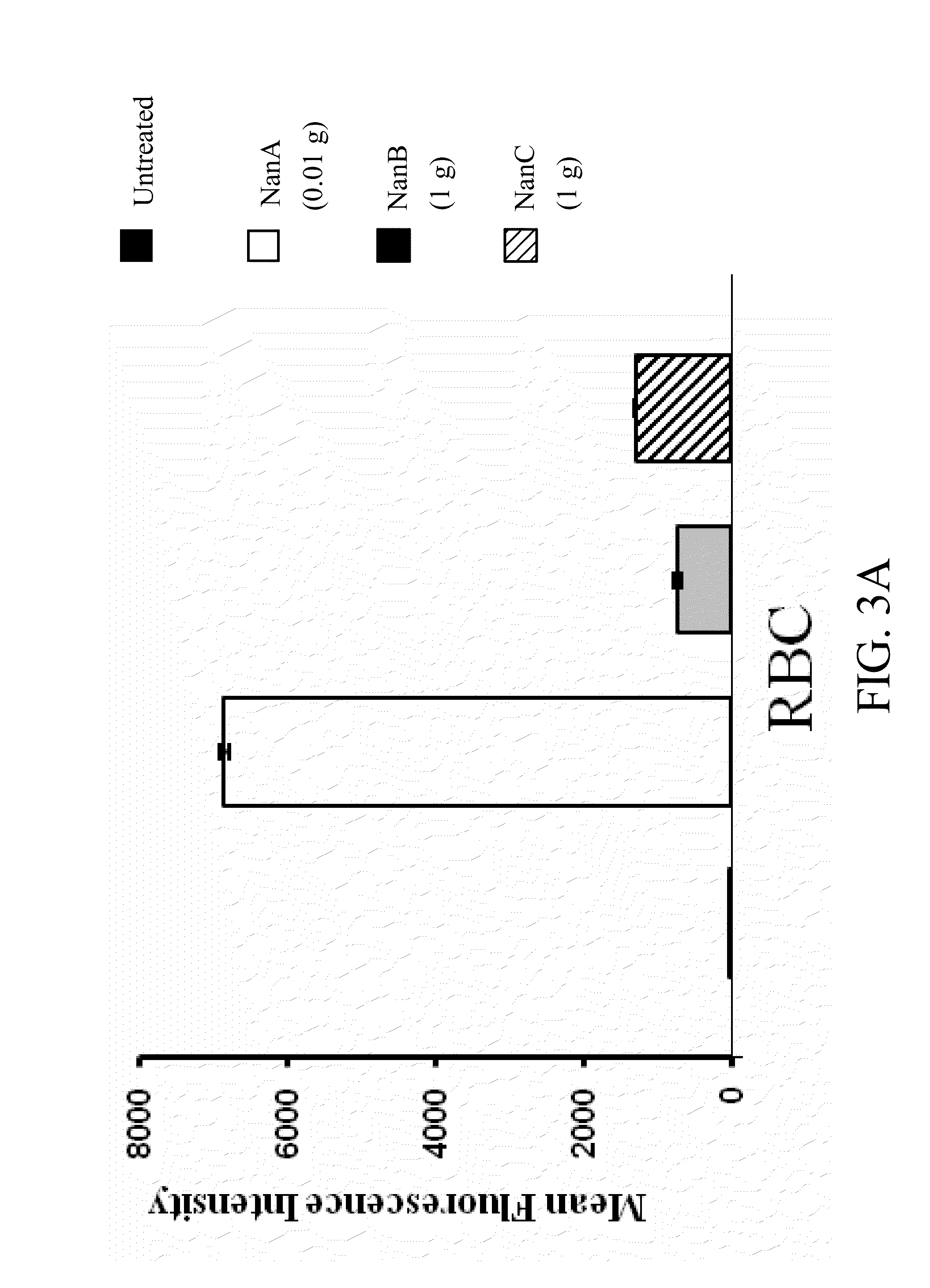
A method of providing protection against pneumococcal infection in a subject is disclosed. The method includes steps of administering to the subject a composition that includes combination of three recombinant pneumococcal neuraminidases: NanA, NanB, and NanC of S. pneumoniae strains CGSP14, wherein administration of the recombinant pneumococcal neuraminidases elicits an immune response to S. pneumoniae, and treats the subject. In one embodiment, the method further includes a step of adding adjuvants to enhance the immune response. The method also includes a step of using passive antibodies, wherein said passive antibodies are anti-neuraminidase antibodies generated from neuraminidases-immunized humanized animals: NanA, NanB, and NanC. Meanwhile, this invention also provides a method for the molecular diagnosis of pneumococcal infection.
Owner:CHUNG GUNG MEDICAL FOUNDATION LINKOU BRANCH
New virulence factors of streptococcus pneumoniae
The present invention provides proteins / genes, which are essential for survival, and consequently, for virulence of Streptococcus pneumoniae in vivo, and thus are ideal vaccine candidates for a vaccine preparation against pneumococcal infection. Further, also antibodies against said protein(s) are included in the invention.
Owner:STICHTING KATHOLIEKE UNIV MORE PARTICULARLY THE RADBOUD UNIV NIJMEGEN MEDICAL CENT
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS9347944B2Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
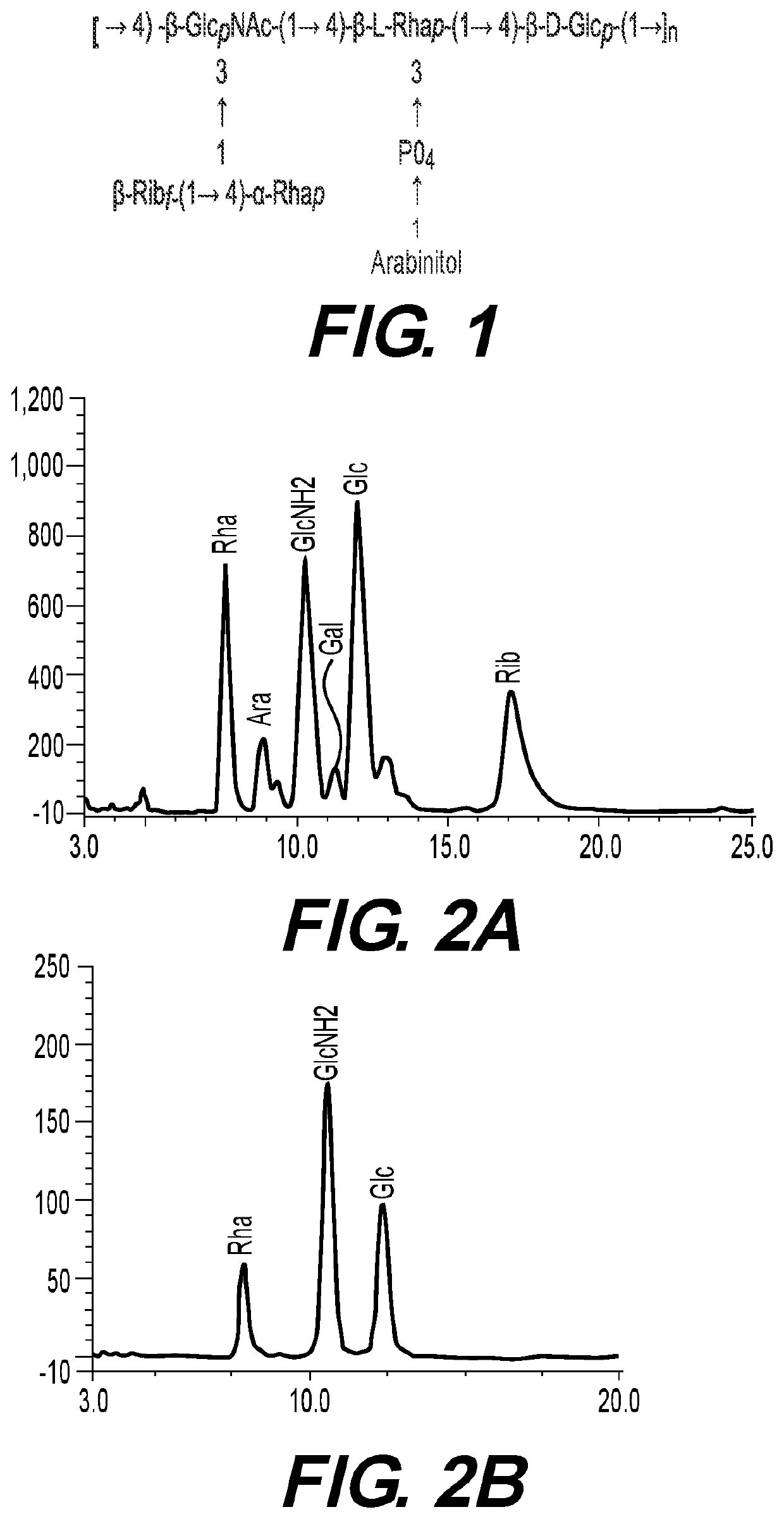
Streptococcus pneumoniae vaccine and preparation method thereof
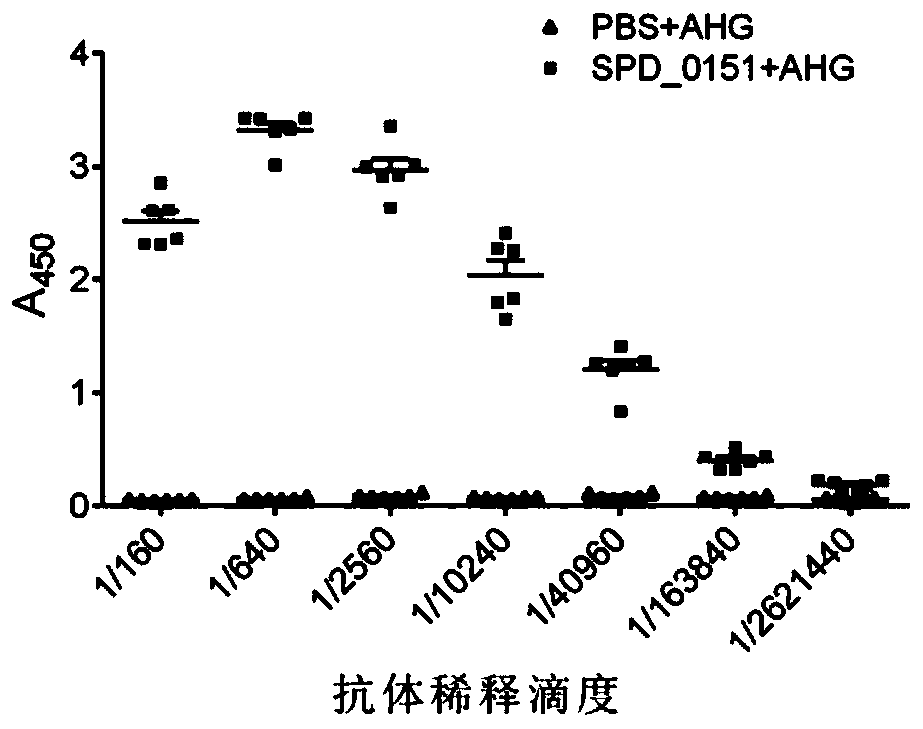
ActiveCN110898217AAvoid infectionAntibacterial agentsBacterial antigen ingredientsMicrobiologyTGE VACCINE
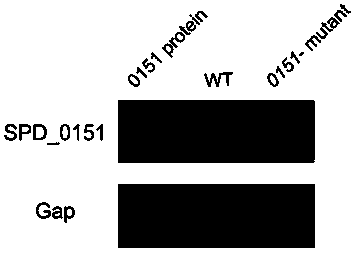
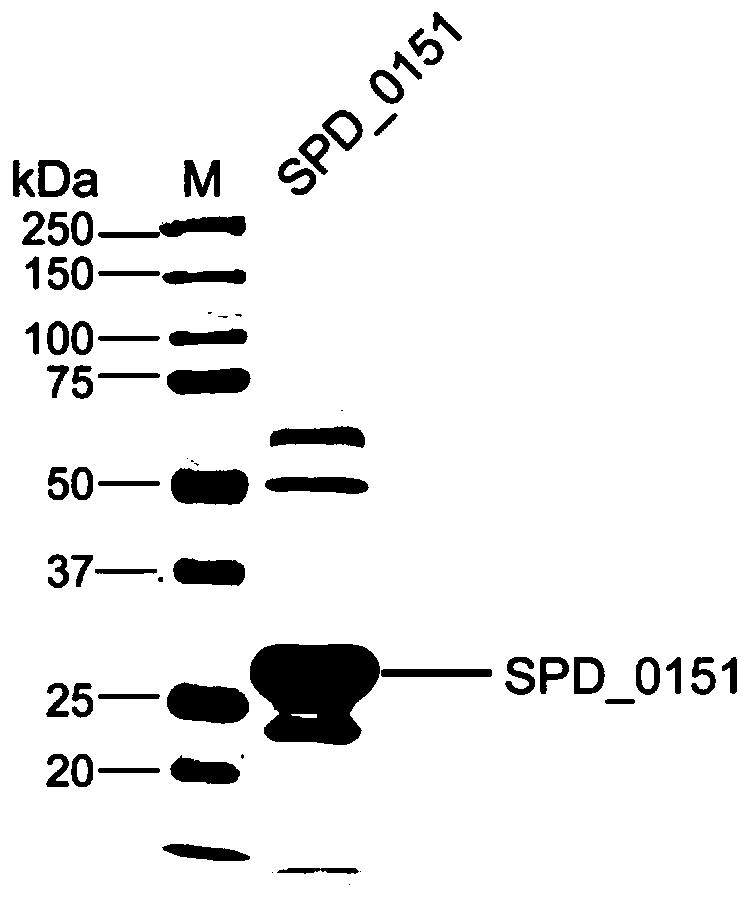
The invention belongs to the field of biological products, and discloses a streptococcus pneumoniae vaccine, which comprises SPD_0151 protein and an immunologic adjuvant, and the amino acid sequence of the SPD_ 0151 protein is SEQ ID NO.1. The invention also discloses a preparation method of the SPD_0151 protein, which comprises the following steps: inserting spd_0151 gene which contains no signalpeptide sequence into a recombinant plasmids, introducing the recombinant plasmids into expression bacteria, inducing expression, and purifying to obtain the SPD_0151 protein, wherein the sequence ofthe spd_0151 gene is shown as SEQ ID NO.2. The streptococcus pneumoniae vaccine has a good protection effect on challenge of streptococcus pneumoniae strains of different subtypes, and can effectively enhance the resistance to streptococcus pneumoniae.
Owner:遵义医科大学珠海校区
Modulating production of pneumococcal capsular polysaccharide
The invention relates to a method of assessing whether a test compound is useful for alleviating a pneumococcal infection in an animal. This method comprises comparing (a) the degree of phosphorylation of CpsD in pneumococcal cells maintained in the presence of the test compound and (b) the degree of phosphorylation of CpsD in the same type of cells maintained in the absence of the test compound.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
A special diluent for detecting Streptococcus pneumoniae in urine samples
The invention relates to a special diluent for detecting Streptococcus pneumoniae in urine samples. The diluent is composed of five components: borax, 1307Prill, ε-polylysine, C-reactive protein polyclonal antibody, and purified water. The content of each component is as follows: borax as component I content is 50mM ~ 100mM, 1307Prill as component II content is 0.5% ~ 1%, ε‑polylysine as component III content is 0.5% ~ 1%, C‑reaction The content of protein polyclonal antibody as component IV is 10ug / ml-20ug / ml, and the rest is purified water. The diluent mixed with the urine sample at a volume ratio of 1:9 can effectively inhibit the interference of the C-reactive protein in the urine sample on the detection results of Streptococcus pneumoniae, significantly improve the detection rate of Streptococcus pneumoniae, and play an important role in the accurate detection of urine Streptococcus pneumoniae in fluid samples is of great significance and has good clinical application value.
Owner:HANGZHOU BEACONLAB BIOTECH CO LTD
Immunogenic compositions for use in pneumococcal vaccines
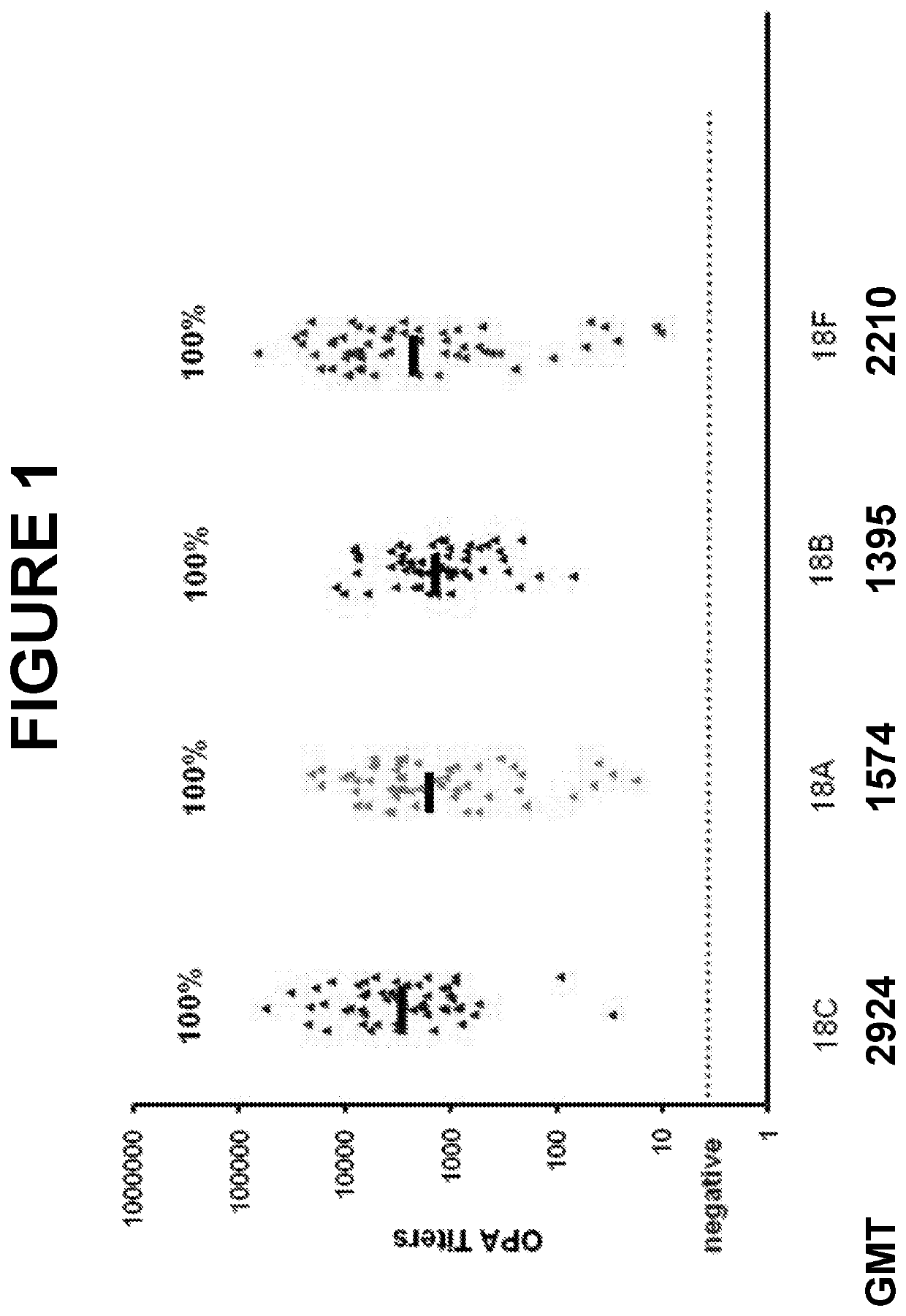
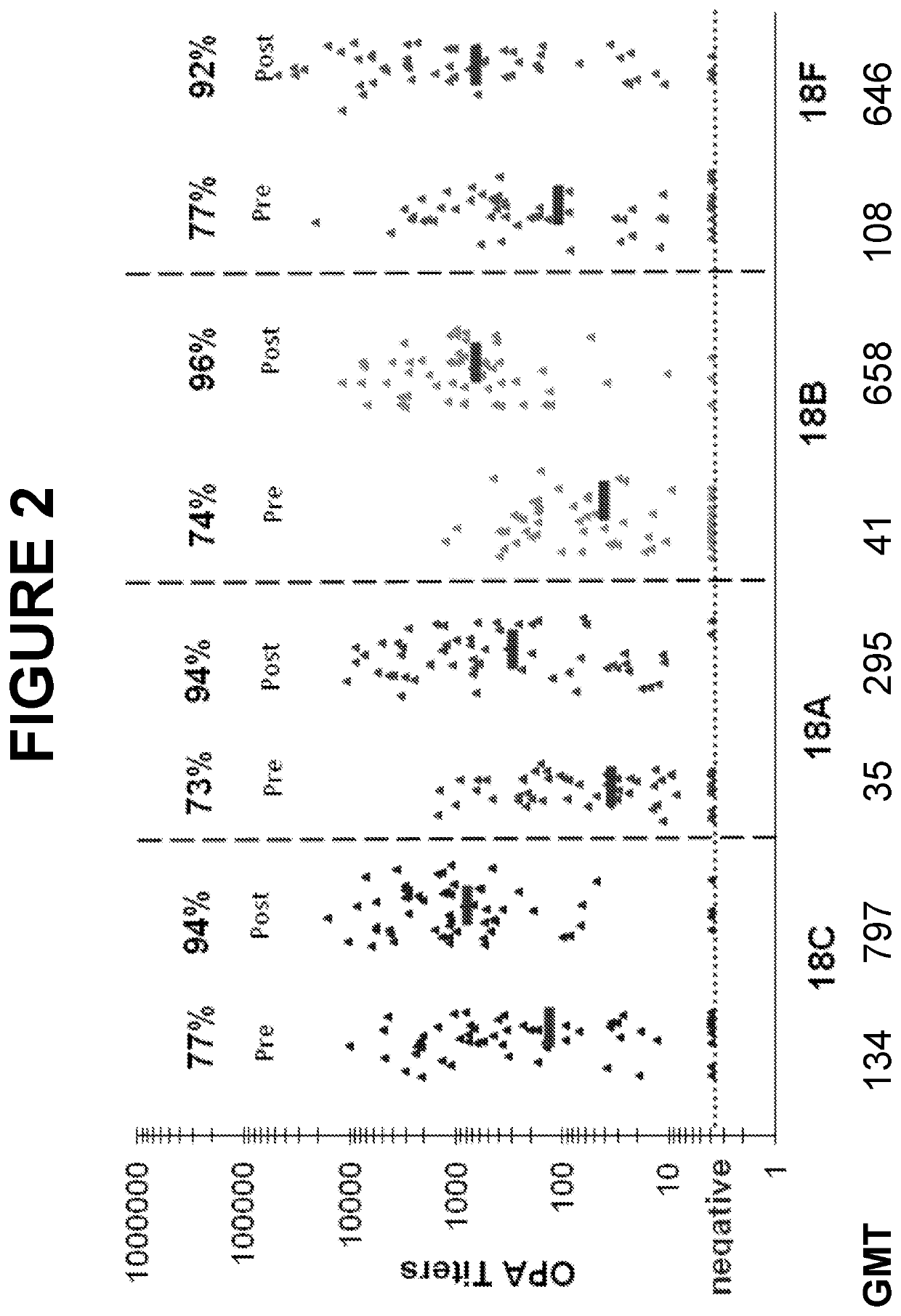
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 18, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
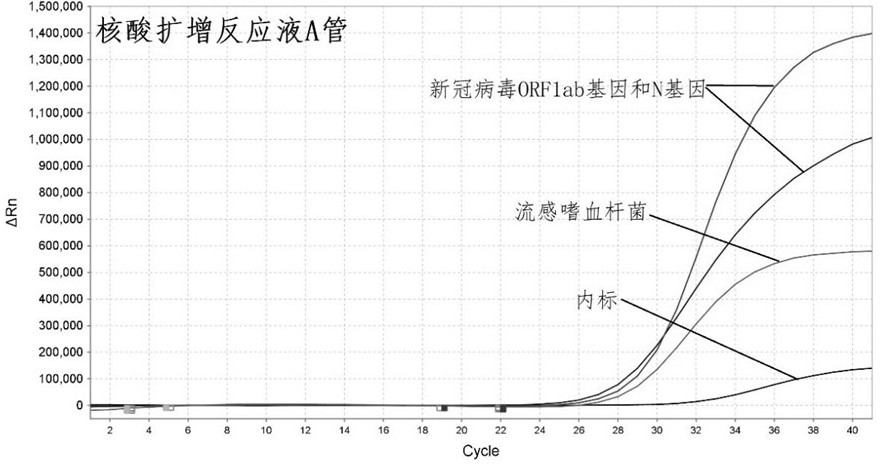
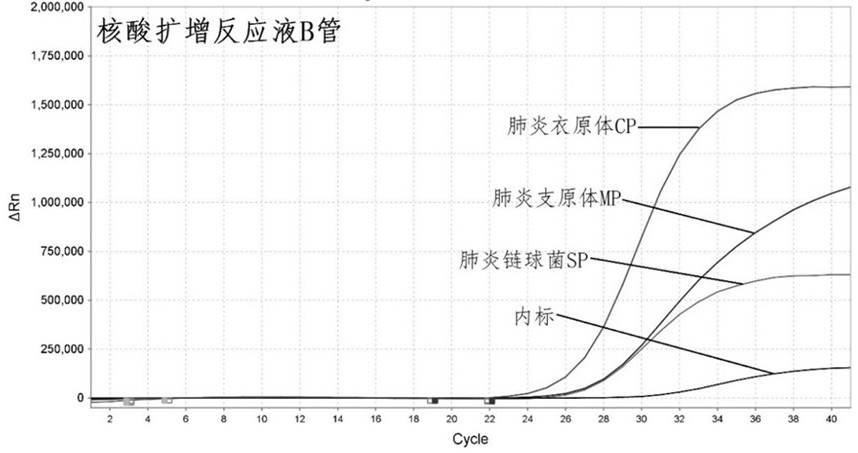
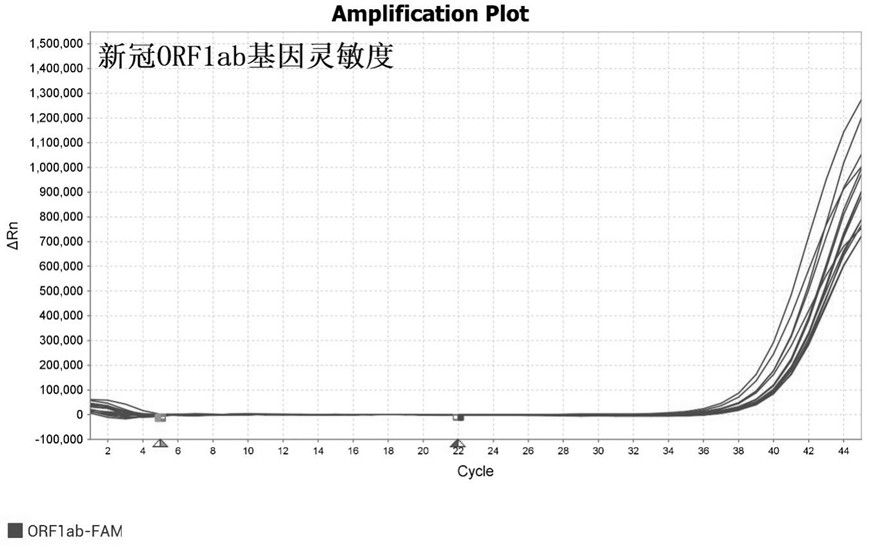
Primer-probe combination and detection kit for detecting new coronavirus and pneumonia pathogenic bacteria
ActiveCN113862400AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesBacilliInfluenza a
The invention discloses a primer-probe combination and detection kit for detecting new coronavirus and pneumonia pathogenic bacteria. A primer pair for detecting a new coronavirus ORF1ab gene is shown as SEQ ID NO: 1-2, and a probe is shown as SEQ ID NO: 3; a primer pair for detecting a new coronavirus N gene is shown as SEQ ID NO: 4-5, and a probe is shown as SEQ ID NO: 6; and a primer pair for detecting haemophilus influenzae is shown as SEQ ID NO: 7-8, and a probe is shown as SEQ ID NO: 9. A primer pair for detecting streptococcus pneumoniae is shown as SEQ ID NO: 10-11, and a probe is shown as SEQ ID NO: 12; a primer pair for detecting mycoplasma pneumoniae is shown as SEQ ID NO: 13-14, and a probe is shown as SEQ ID NO: 15; and a primer pair for detecting chlamydia pneumoniae is shown as SEQ ID NO: 16-17, and a probe is shown as SEQ ID NO: 18. The primers and the probes provided by the invention have good sensitivity and specificity, and can be efficiently applied to screening and triage of new coronavirus infection and community-acquired pneumonia infection by applying the primers and the probes to the detection kit.
Owner:上海思路迪医学检验所有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com