Application of L-rhamnose antibody in preparation of medicine for preventing and/or treating drug-resistant bacterial infection
A technology of drug-resistant bacteria and rhamnose, which is applied in the field of medicine, can solve the problems of staying in the experimental research stage, the difficulty of developing antibiotics, and the weak and incapable of bacterial invasion, so as to achieve strong inhibition and killing ability and improve L-rhamnose antibody Level, good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
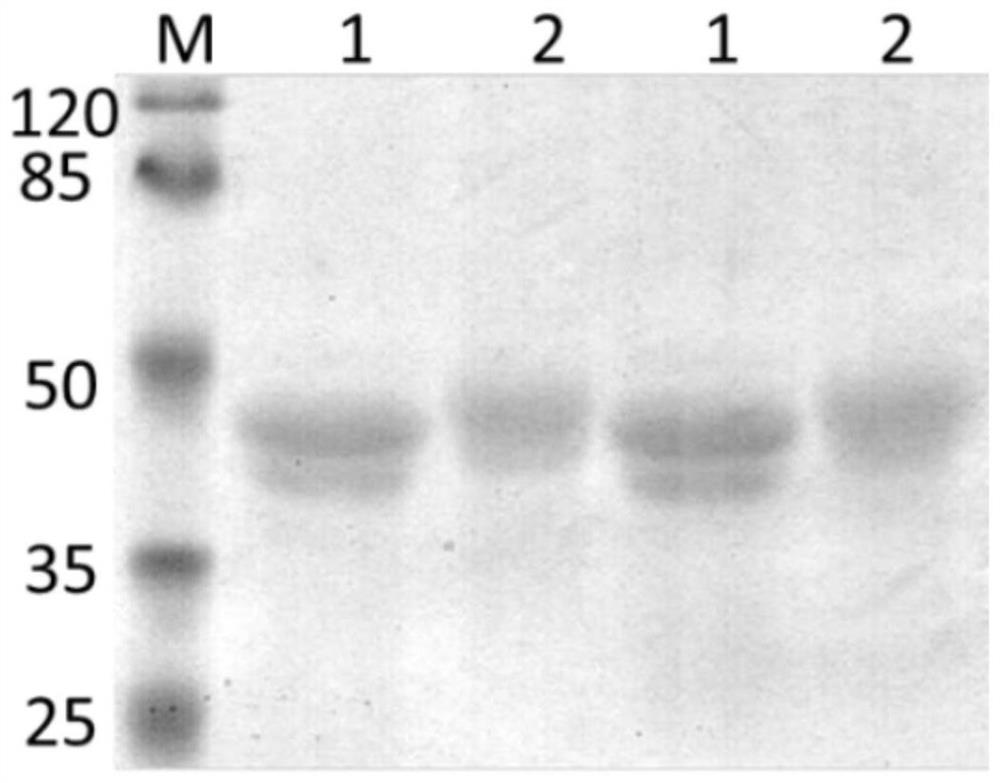
[0084] The preparation method steps of human source L-rhamnose natural antibody are as follows:
[0085] (1) Synthesis of L-rhamnose-NH 2
[0086] 1 mol of L-rhamnose was protected by acetyl group and 1.1 mol of thiophenol was glycosylated to obtain intermediate product 1, and then 1 mol of intermediate product 1 was connected with 2 mol of 3-azidopropanol to obtain intermediate product 2. Product 2 was protected by deacetylation and reduced to obtain L-rhamnose-NH 2 ;
[0087]
[0088] (2) Preparation of L-rhamnose affinity chromatography column
[0089] Take the NHS-activated agarose gel column, wash the column with 10 column volumes of pre-cooled, 1mM HCl, and wash the column with 5 column volumes of 0.2M NaHCO 3 , 0.5M NaCl solution (pH=8.3) to equilibrate the column; add 2 mL of L-rhamnose-NH with a concentration of 10 mg / mL 2 After that, mix evenly, react with slow shaking at room temperature for 4 hours, continue to add 3 column volumes of 0.1M Tris-HCl solution...
Embodiment 2
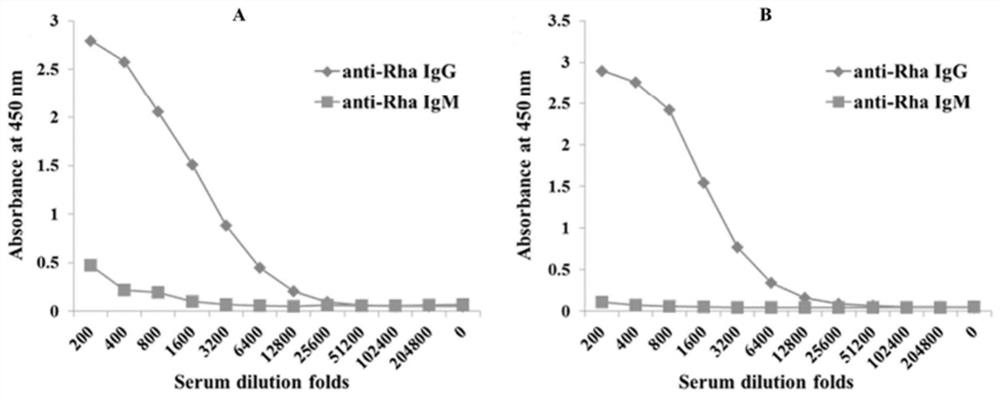
[0094] Example 2 Preparation of mouse L-rhamnose monoclonal antibody
[0095] The preparation method step of mouse source L-rhamnose monoclonal antibody is as follows:
[0096] (1) Synthesis of L-rhamnose-NHS
[0097] 1mol of L-rhamnose-NH 2 Reaction with 1.1 mol of succinyl gave intermediate 3, which was activated by 1.1 mol of 2-succinimidyl-1,1,3,3-tetramethylurea tetrafluoroborate to give L-rhamnose-NHS;
[0098]
[0099] (2) Preparation of L-rhamnose-OVA
[0100] Dissolve 10mg of L-rhamnose-NHS in 1mL 3×PBS buffer (pH=7.4); another 10mg of OVA was dissolved in 1mL 3×PBS buffer (pH=7.4); Stir for 1 hour; transfer the above reaction system to a 3kDa ultrafiltration tube, centrifuge at 4°C for 30 minutes at 3,500 rpm, add an appropriate amount of 1×PBS buffer in the tube, and centrifuge at 4°C for 30 minutes at 3,500 rpm, repeat 3 times, The solution obtained in the casing is L-rhamnose-OVA;
[0101] (3) Preparation of mouse splenocyte suspension
[0102] Take five...
Embodiment 3
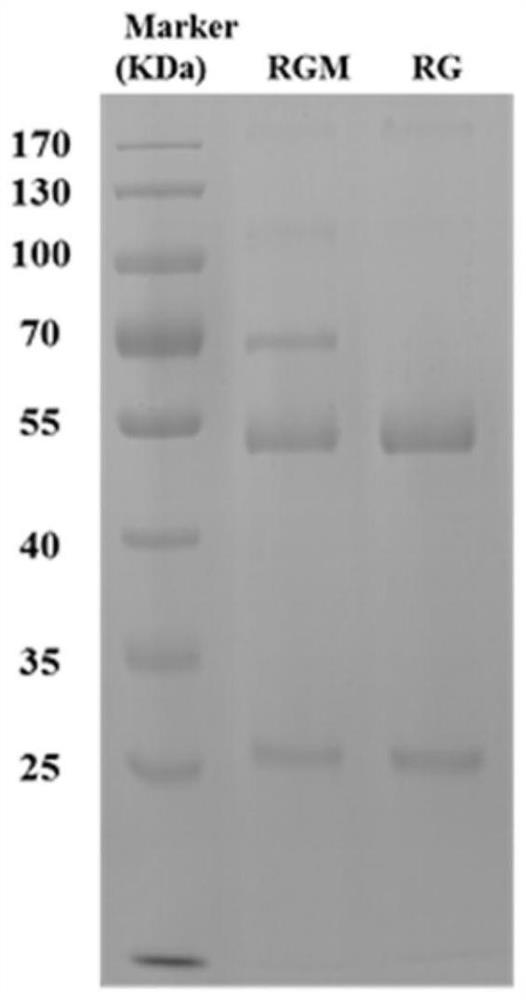
[0110] Example 3 Bactericidal effect test of human L-rhamnose natural antibody
[0111] Bacterial lipopolysaccharide extraction and SDS-PAGE / silver staining detection, the specific method steps are as follows:
[0112] (1) Extracting bacterial lipopolysaccharide: inoculate 5 μL of glycerol bacteria into 5 mL of LB liquid medium, and cultivate overnight at 37° C. at 220 rpm; centrifuge to collect overnight cultured cells, and extract lipopolysaccharide with a lipopolysaccharide extraction kit; Glycerol bacteria are Escherichia coli or Pseudomonas aeruginosa;
[0113] (2) SDS-PAGE / silver staining detection: Take 5 μL of the extracted bacterial lipopolysaccharide, add 1 μL 6×SDS sample buffer, mix well and boil for 6 minutes; the obtained sample is used for 12% SDS-PAGE electrophoresis; the electrophoresis is over After that, in fixative solution (35mL H 2 O, 15mL acetic acid, 50mL isopropanol), shake slowly for 2 hours, then wash the gel twice with 200mL 7.5% glacial acetic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com