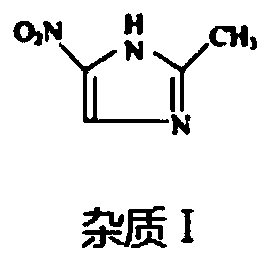
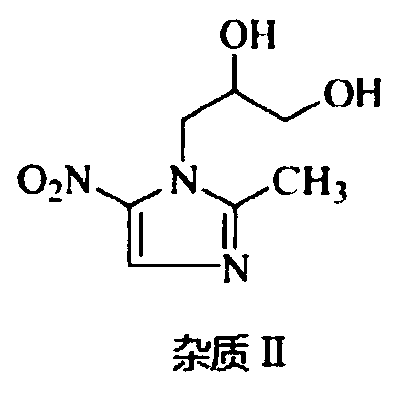
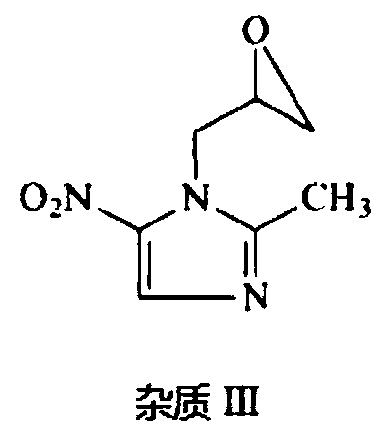
Solvent system capable of effectively dissolving ornidazole or levorotatory ornidazole and injection thereof
A technology of levoornidazole and ornidazole, which is applied to the more stable ornidazole injection or levoornidazole injection and the field of preparation thereof, and can solve the problem of excessive use of propylene glycol, control of impurity growth, easy crystallization at low temperature, etc. problem, to achieve the effect of reducing the amount of solvent, easy crystallization, and reducing the amount of solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0062] Embodiment 1~11: Different organic systems are to the solubility of ornidazole or left-ornidazole (1) (room temperature)
[0063] Experimental procedure: first mix various solvents in proportion, then add excess ornidazole or left ornidazole, stir and dissolve at room temperature for 20 minutes, remove undissolved drugs by filtration with a 0.22 micron microporous membrane, and use spectrophotometry ( 318nm) to measure the absorbance of the solution, and calculate the drug solubility according to the standard curve. Note: the amount of each component is parts by weight, the same below.
[0064] Table 1: Examples 1-11
[0065]
Embodiment 27~36
[0081] Embodiment 27~36: low temperature stability test (1)
[0082] Table 5: Examples 27-36
[0083]
Embodiment 37~46
[0084] Embodiment 37~46: low temperature stability test (2)
[0085] Table 6: Examples 37-46
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com