Preparation method and application of double non-noble metal catalyst with high specific surface area and high defects
A high specific surface area, non-precious metal technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, non-metallic elements, etc., can solve the problems of inability to use synthetic pure metal catalysts, high energy consumption, etc. , to achieve good cycle activity, high hydrogen production rate, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
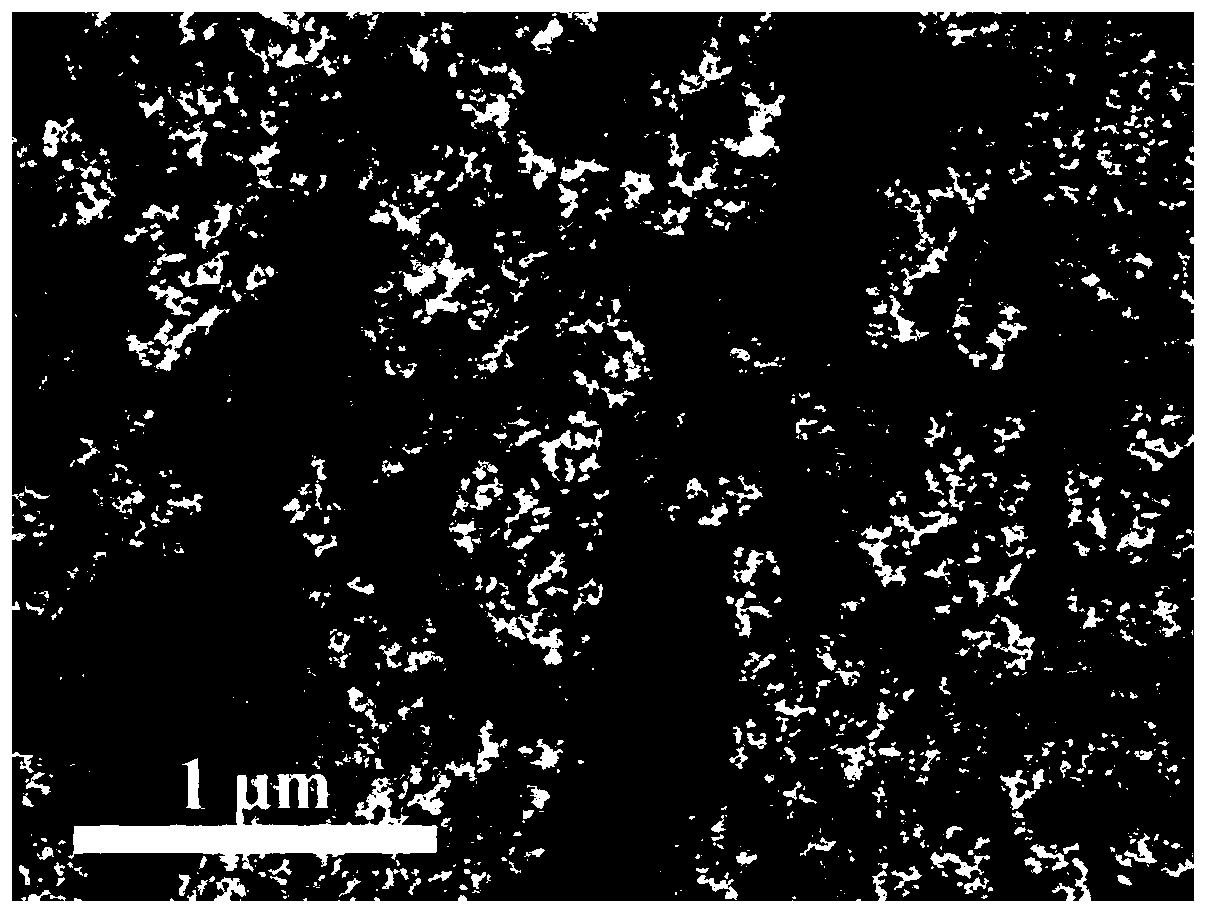
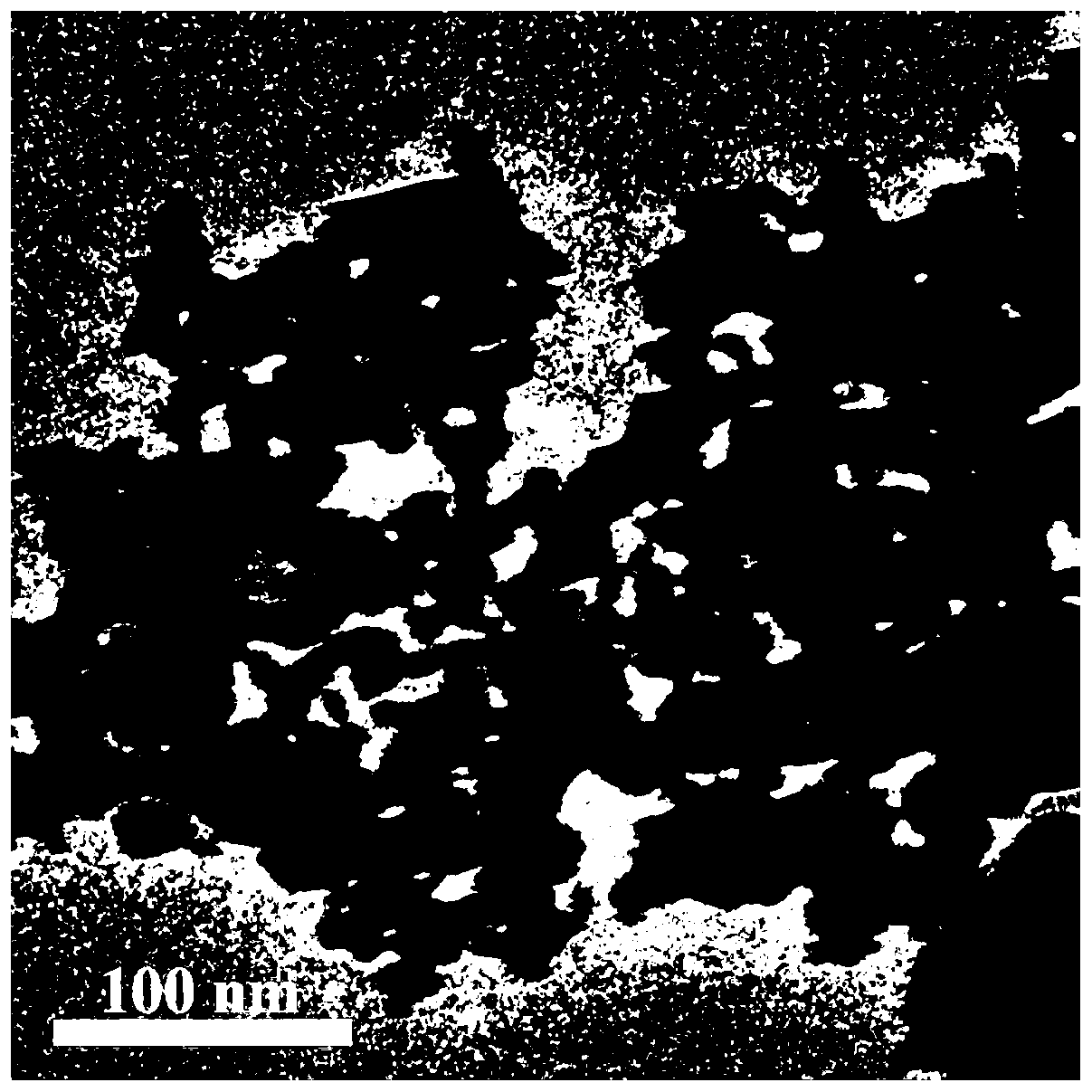
Image
Examples
Embodiment 2
[0044] In the step (2) of Example 1, the reaction time of the acid etching mixture at 20° C. was changed from 5 minutes to 10 minutes, and other experimental operations and drug dosages were the same as in Example 1.
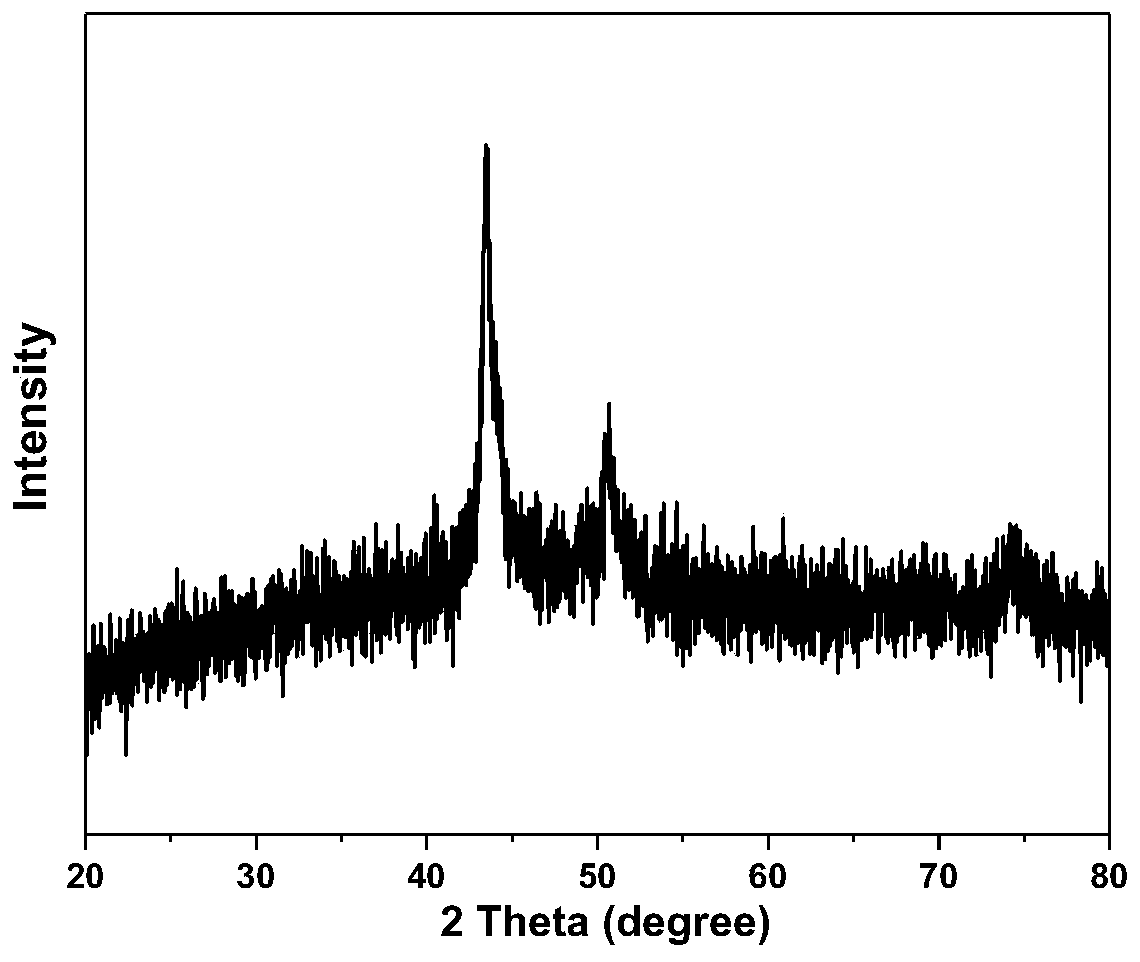
[0045] The XRD spectrogram of the catalyst of embodiment 2 gained ( Figure 6 ) shows that the catalyst is still a cobalt-copper alloy structure, and the intensity and position of the diffraction peaks do not change significantly, indicating that the catalyst structure does not change with the increase of acid etching time. The prepared cobalt-copper catalyst with high specific surface area and high defects catalyzed the hydrolysis of ammonia borane in the experiment of dehydrogenation through the multi-channel micro gas flowmeter (model Rock-Solar-Ⅰ) to test the hydrogen desorption diagram ( Figure 7 ), wherein the catalytic hydrogen desorption test temperature is 25 ° C, the amount of catalyst used is 5 mg, and the ammonia borane aqueous solution adopts a con...
Embodiment 1
[0047] The dosages of cobalt chloride and cupric chloride in Examples 10-14 are shown in Table 1, and other experimental operations and drug dosages are the same as in Example 1.
[0048] Cobalt chloride and cupric chloride consumption in the embodiment 10-14 of table 1
[0049]
Embodiment 1
[0050] The catalysts obtained in Examples 10-14 have the same structure and appearance as in Example 1, only the content of metal cobalt and metal copper in the catalyst can be changed.
[0051] In Examples 15-17, the precursors of non-noble metals were replaced with ferrous chloride, nickel chloride, zinc chloride, cobalt chloride and other chlorides in Table 2. Other experimental operations and drug consumption are the same as those in Examples 1 is the same.
[0052] Cobalt chloride and other chloride consumptions in table 2 embodiment 15-17
[0053]
[0054] The catalysts obtained in Examples 15-17 have the same morphology as in Example 1, and also form double non-precious metal alloy catalysts, which have good activity in catalyzing ammonia borane to produce hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com