Preparation method of anti-RET mutant protein monoclonal antibody variable region sequence
A technology of monoclonal antibody and variable region, applied in anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, immunoglobulin, etc. As a result, there were no RET protein point mutations, monoclonal antibodies could not detect protein mutant types, etc., and achieved the effect of convenient and fast screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of the variable region sequence of the anti-RET mutant protein monoclonal antibody, the specific steps are as follows:
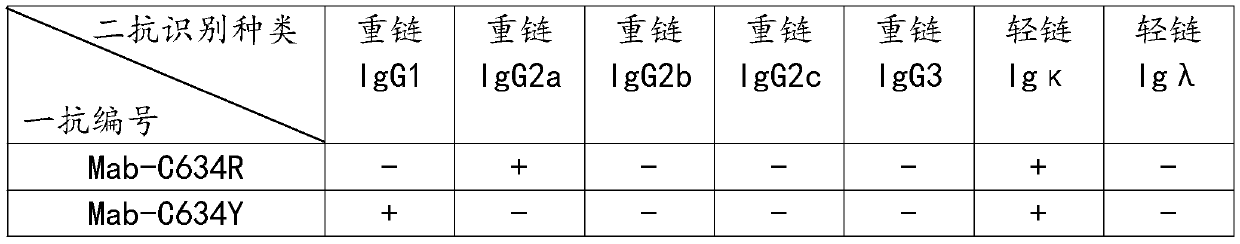
[0036] Step S1: For the C634 site of the RET protein, design and synthesize the original polypeptide C634, mutate the C634 site, and synthesize the mutant polypeptides C634R, C634Y, C634S, C634G, C634W, C634F, and use glutaraldehyde as a cross-linker for the mutant polypeptides respectively. The agent is coupled with the carrier protein KLH to prepare the antigen, and the antigen is mixed to prepare the antigen mixture and emulsified with Freund's adjuvant to obtain the emulsified antigen;
[0037] Step S2: Construct mutant polypeptide expression sequences C634R, C634Y, C634S, C634G, C634W, C634F respectively to the amino terminus of purple fluorescent protein mcherry, and clone them into pet22b expression vector. After prokaryotic expression and purification, mutant proteins C634R-mcherry, C634Y- After mcherry, C634S-mcherry, C...
Embodiment 2
[0042] Antibody WB detection verification, the specific steps are as follows:
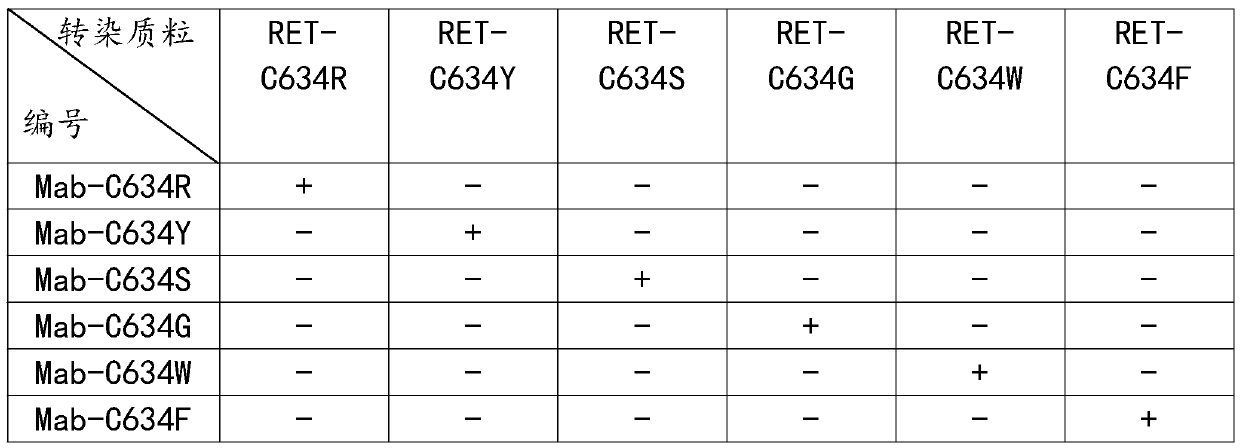
[0043] Step S1: respectively construct pcDNA3.1-RET-C634R, pcDNA3.1-RET-C634Y, pcDNA3.1-RET-C634S, pcDNA3.1-RET-C634G, pcDNA3.1-RET-C634W, pcDNA3.1-RET -C634F full-length protein eukaryotic expression vector, using endotoxin-removing plasmid extraction kit to extract plasmids to obtain 6 kinds of plasmids;
[0044] Step S2: revive HeLa cells, culture HeLa cells in an incubator to log phase, press 1×10 5 Cells / mL were passaged into a 6-well plate, and the addition amount was 2 mL / well. After the 6-well plate was incubated in an incubator for 24 hours, the 6 plasmids prepared in step S1 were respectively transfected with liposome transfection reagent. After reaching Hela cells and culturing for 48 hours, the cells were digested with trypsin, and the cells were collected into EP tubes, washed three times with PBS, and 100 μL of cell lysate was added to the EP tubes. Under the conditions of 4 °C and ...
Embodiment 3
[0051] Antibody IF detection and verification, the specific steps are as follows:
[0052] Step S1: respectively construct pcDNA3.1-RET-C634R, pcDNA3.1-RET-C634Y, pcDNA3.1-RET-C634S, pcDNA3.1-RET-C634G, pcDNA3.1-RET-C634W, pcDNA3.1-RET -C634F eukaryotic expression vector, using endotoxin-removing plasmid extraction kit to extract plasmids to obtain 6 kinds of plasmids;
[0053] Step S2: revive HeLa cells, culture HeLa cells in an incubator to log phase, press 2 × 10 4 Cells / mL were passaged into a 24-well plate, and the addition amount was 1 mL / well. After the 24-well plate was cultured in an incubator for 24 hours, the 6 plasmids prepared in step S1 were respectively transfected with liposome transfection reagent. Transfected into Hela cells and cultured for 48h to obtain transfected cells;
[0054] Step S3: Aspirate the medium in the wells of the 24-plate in step S2, rinse the transfected cells prepared in step S2 twice with PBS, aspirate the PBS, and add 2-PBS prepared to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com