Acceptor material based on benzoxadiazole and preparation method and application of acceptor material
A technology of benzoxo group and acceptor materials, which is applied in the manufacture of semiconductor/solid-state devices, electric solid-state devices, semiconductor devices, etc., can solve the problem of polydispersity of molecular weight distribution and poor repeatability between batches, difficulty in energy level regulation, and invariable Stability and other issues, to achieve the effect of improving photoelectric conversion efficiency, narrow optical bandgap, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
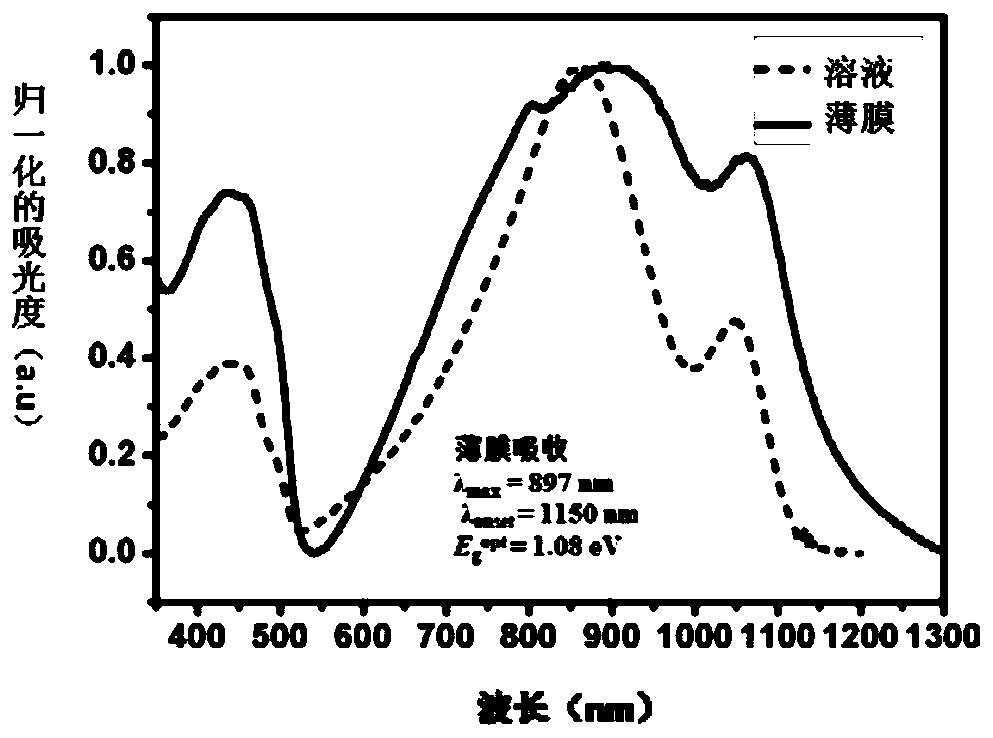
Image
Examples
preparation example Construction
[0061] The embodiment of the present invention also provides a preparation method of a benzoxadiazole-based acceptor material, comprising the following steps:
[0062] S10. Obtaining aldehyde compounds and electron-withdrawing compounds containing benzoxadiazole units;
[0063] S20. Obtain a weakly basic catalyst, dissolve the catalyst, the aldehyde compound and the electron-withdrawing compound in an organic solvent, and reflux at 60°C to 70°C for 12 to 24 hours to obtain a benzene-based Acceptor materials for oxadiazoles;
[0064] Wherein, the aldehyde compound containing benzoxadiazole unit is:
[0065]
[0066] The electron-withdrawing compound is selected from any one of the following compounds:
[0067]
[0068] Among them, A is selected from oxygen group elements, R 1 from C 16 ~C 30 branched chain alkyl, and the C 16 ~C 30 In the branched chain alkyl group, a branch is formed on at least one of the first three carbons adjacent to the N atom, R 2 Selected ...
Embodiment 1
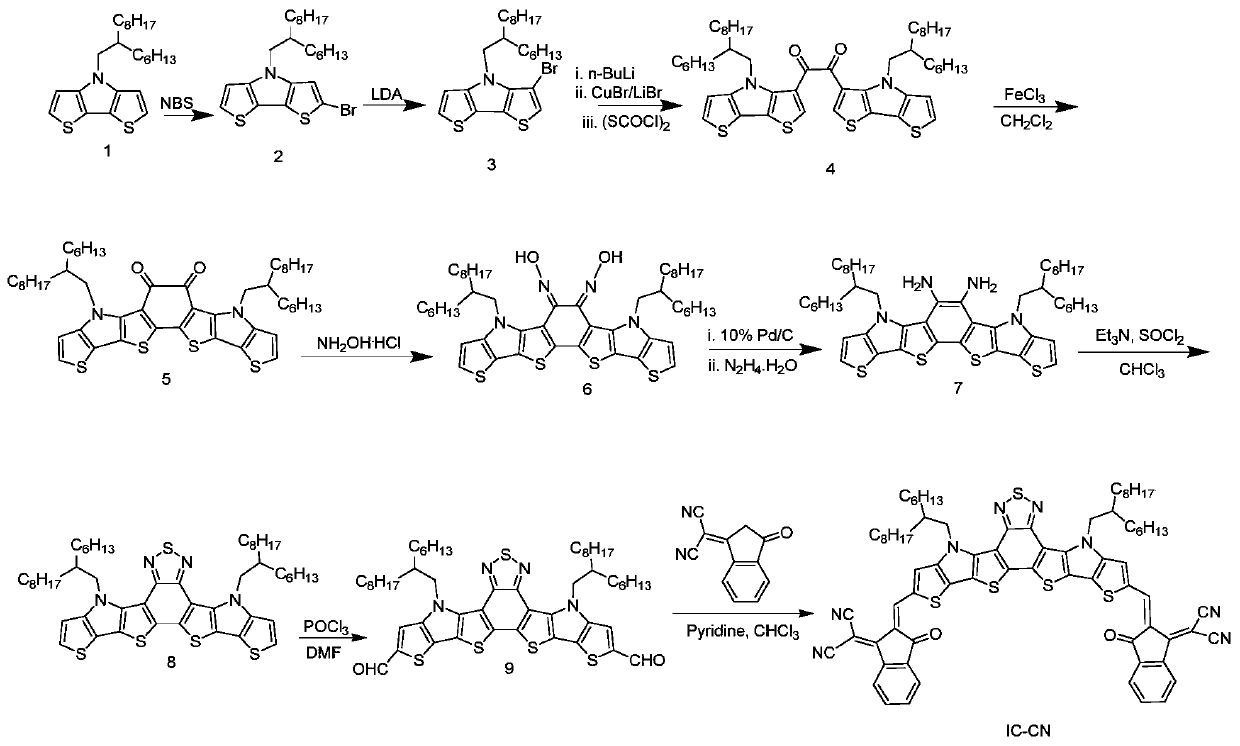
[0119] A kind of preparation method of acceptor material based on benzothiadiazole, as attached figure 1 The synthetic route is shown as:
[0120] S10. to Aldehyde compounds containing benzothiadiazole units were synthesized as starting compound 1.
[0121] ①Dissolve compound 1 (5.0g, 12.4mmol) in 100mL of tetrahydrofuran, stir at room temperature, add N-bromosuccinimide (2.21g, 12.4mmol) in batches, stir and react at room temperature for 12 hours, then add 40 mL of saturated sodium sulfite solution was extracted three times with dichloromethane, and then dried over anhydrous sodium sulfate. Purify with silica gel column, use petroleum ether as eluent, and concentrate in vacuo to obtain a light yellow liquid, namely compound 2, with a yield of 90.4%. MS (EI, m / z) 481.2;
[0122] ② Dissolve compound 2 (4.0g, 8.3mmol) in 60mL of tetrahydrofuran, then cool down to -78°C, stir, and gradually add 2M lithium diisopropylamide solution 4.35mL (8.7mmol) dropwise, react at -78°C A...
Embodiment 2
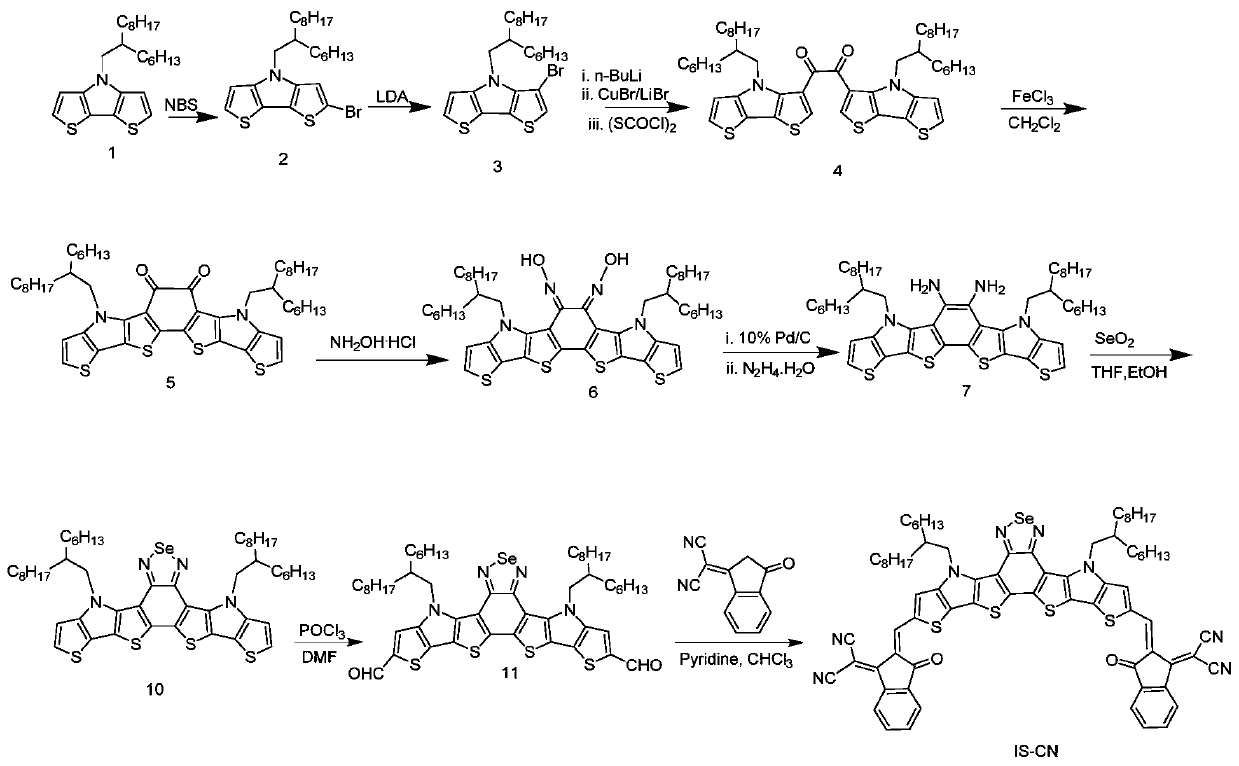
[0133] A kind of preparation method of acceptor material based on benzoselenadiazole, as attached figure 2 The synthetic route is shown as:
[0134] S10. to Aldehyde compounds containing benzoselenadiazole units were synthesized as starting material compound 1.
[0135] ①Dissolve compound 1 (5.0g, 12.4mmol) in 100mL of tetrahydrofuran, stir at room temperature, add N-bromosuccinimide (2.21g, 12.4mmol) in batches, stir and react at room temperature for 12 hours, then add 40 mL of saturated sodium sulfite solution was extracted three times with dichloromethane, and then dried over anhydrous sodium sulfate. Purify with silica gel column, use petroleum ether as eluent, and concentrate in vacuo to obtain a light yellow liquid, namely compound 2, with a yield of 90.4%. MS (EI, m / z) 481.2;
[0136] ② Dissolve compound 2 (4.0g, 8.3mmol) in 60mL of tetrahydrofuran, then cool down to -78°C, stir, and gradually add 2M lithium diisopropylamide solution 4.35mL (8.7mmol) dropwise, rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com