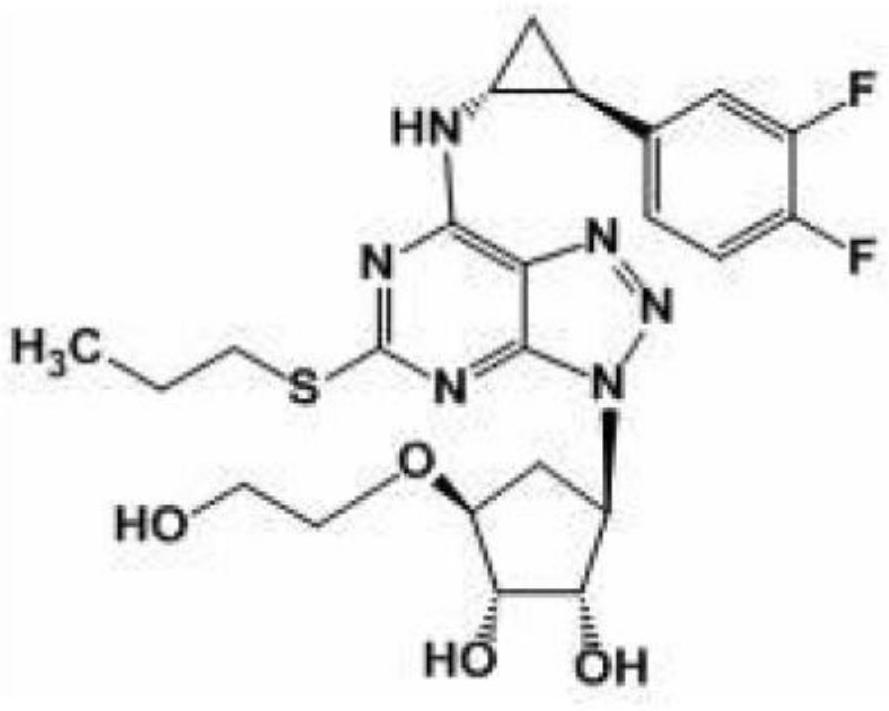
A kind of preparation method of ticagrelor key intermediate iodide
A technology of ticagrelor and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of triphenylphosphine oxide by-product, sodium p-toluenesulfonate by-product, unfavorable industrial production, etc. Yield, the effect that is conducive to production cost control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
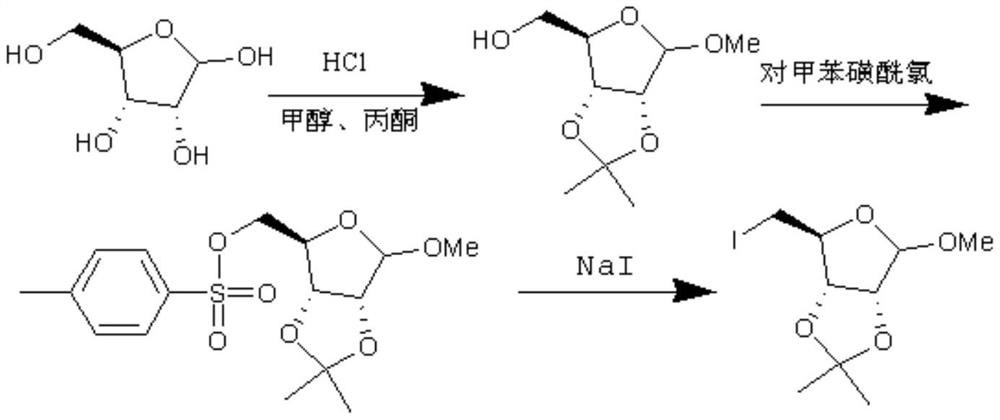
[0039] Synthesis of Intermediate 1
[0040] The specific synthesis steps are as follows: add 100ml of acetone, 100ml of methanol, and 20g of D-ribose (0.133mol) into a 250ml reaction flask, add 2g of thionyl chloride dropwise at a controlled temperature of 10-15°C, and heat up to 25±3°C for 24 hours after adding , the solvent was recovered under negative pressure until no liquid was produced, and 27 g of intermediate 1 was obtained with a yield of 99% and a purity of 90%.
[0041] The yield of intermediate 1 was 99% and the purity was 90%.
[0042] The reaction route is as follows:
[0043]
Embodiment 2
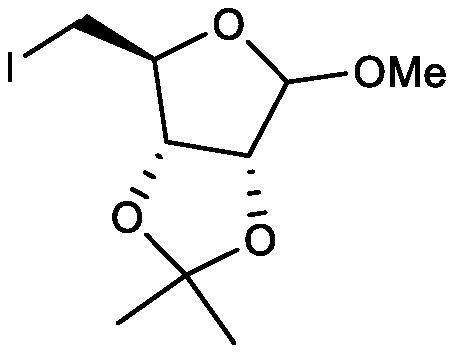
[0045] Synthesis of key intermediate iodides
[0046] The specific synthesis steps are as follows: add 27 g (0.132 mol) of the above-mentioned intermediate 1, 200 ml of acetonitrile, and 29.8 g (0.198 mol) of sodium iodide into a 500 ml reaction flask, nitrogen protection, and dropwise addition of trimethylchlorosilane at a temperature of 20±2°C 21.5g (0.198mol), kept for 1 hour after adding, add 20ml of water, recover the solvent under negative pressure until no liquid is left, add 100ml of water, extract three times with 100ml*3 of toluene, combine the organic layers, and concentrate under negative pressure until no liquid is produced, The key intermediate iodide 35.3g was obtained.
[0047] The yield of the key intermediate iodide was 85% and the purity was 95%.
[0048] The reaction route is as follows:
[0049]
[0050]To sum up, the preparation method of ticagrelor key intermediate iodide of the present invention adopts a two-step method to directly synthesize the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com