Capillary gas chromatography chiral column based on chiral organic molecular cage material
An organic molecule and gas chromatography technology, which is applied in the field of capillary gas chromatography chiral columns based on chiral organic molecular cage materials, can solve the complex preparation process of cyclodextrin derivatives, the high price of gas chromatography chiral columns, and racemization. The problem of not being able to resolve the body is achieved, and the effect of good chiral separation effect, low cost of column preparation and simple preparation method is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
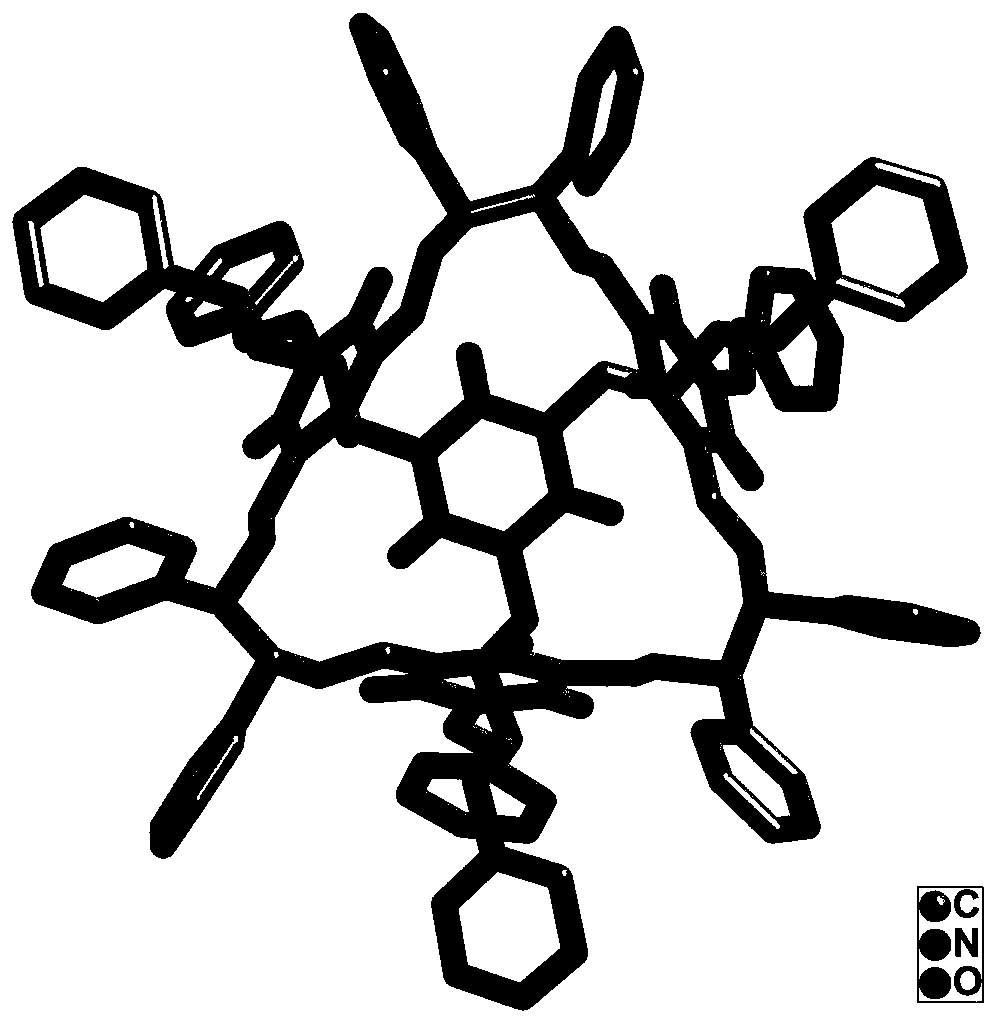
[0023] (1) Synthesis of chiral organic molecular cage materials: Take 0.318g (1.5mmol) (1R,2R)-1,2-diphenyl-1,2-ethylenediamine in a 100mL round bottom flask, add 45mL di Methyl sulfoxide and 5mL water, stir to dissolve; 5 minutes later, add 0.178g (1mmol) 2-hydroxy-1,3,5-triscarbaldehyde to the above solution, seal the entire reaction solution, and stir the reaction at room temperature 2 weeks; during the reaction process, a yellow solid is gradually produced. After 2 weeks of reaction, the reaction solution is filtered, and the obtained solid is washed 3 times with chloroform and water to obtain the chiral organic molecular cage material;
[0024] (2) Roughening treatment of the inner wall of the capillary: Fill the quartz capillary column (30m×0.25mm i.d.) with 1mol / L NaOH solution and keep it for 2 hours to make the inner wall roughened by NaOH reaction corrosion, then rinse the capillary with distilled water The inner wall was washed for 1 hour, then washed with 0.1mol / L ...
Embodiment 2
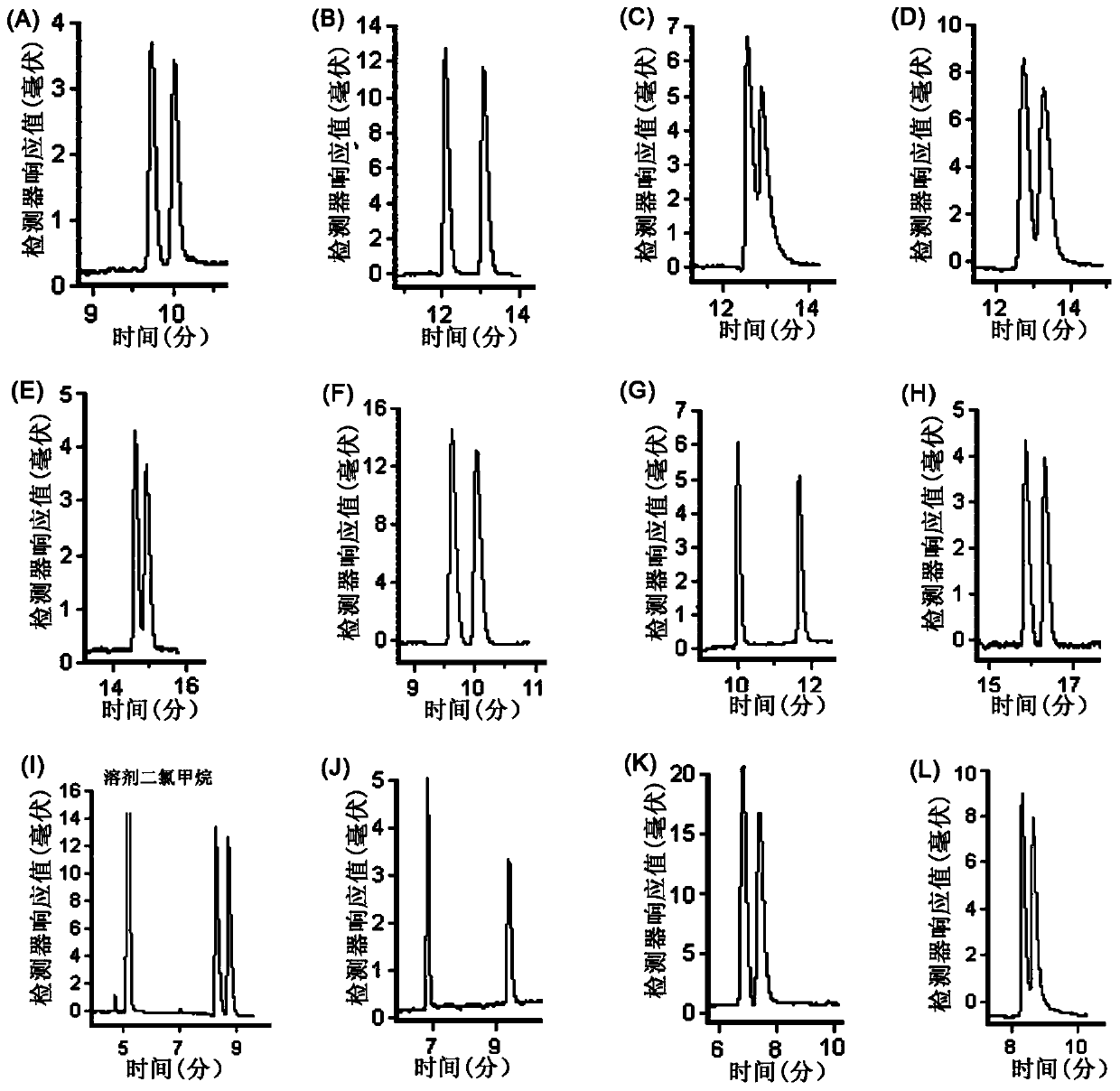
[0027] The capillary column obtained in Example 1 was used to test its resolution effect on chiral compounds. For the split chromatogram see image 3 . image 3 The chiral compounds resolved in and the optimized chromatographic conditions are as follows: (A) 1-phenylethanol, column temperature 157°C, nitrogen linear velocity 15.0cm s -1 ; (B) 2-octanol, column temperature 145°C, nitrogen linear velocity 15.6cm s -1 ; (C) 1,2-butanediol, column temperature 170°C, nitrogen linear velocity 16.7cm s -1 ; (D) 1,2-dichlorobutane, column temperature 120°C, nitrogen linear velocity 15.2cm s -1 ; (E) Epibromopropane, column temperature 120°C, nitrogen linear velocity 16.1cm s -1 ; (F) 1,2-epoxyhexane, column temperature 125°C, nitrogen linear velocity 16.4cm s -1 ; (G) ethyl 3-hydroxybutyrate, column temperature 150°C, nitrogen linear velocity 16.7cm s -1 ; (H) γ-decalactone, column temperature 205 ℃, nitrogen linear velocity 14.3cm s -1 ; (I) 3-hydroxy-2-butanone, column temper...
Embodiment 3
[0030] Some chiral compounds (such as 2-octanol, 1,2-dichlorobutane, epoxybromohydrin, 3-hydroxybutyrate) are treated with the capillary column obtained in Example 1 and the existing commercial product β-DEX 120 capillary gas chromatography column Acetate methyl ester, 3-hydroxybutyrate ethyl ester, γ-decalactone, propylene glycol monomethyl ether and sec-butyl methyl ether) for resolution research, and compare their chiral resolution effects. See the attached chromatogram for the comparison resolution effect Figure 4 .
[0031] Depend on Figure 4 Known: 2-octanol, 1,2-dichlorobutane, epibromohydrin, methyl 3-hydroxybutyrate, ethyl 3-hydroxybutyrate, γ-decalactone, propylene glycol monomethyl ether and sec-butyl methyl Ether is not resolved on the existing commercial β-DEX 120 capillary column, but the chromatographic column of the present invention can better resolve the above-mentioned chiral compounds, showing that the chromatographic column of the present invention is b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com