Synthetic method of cefathiamidine
A technology of cefathiamidine and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of high reaction activity of bromoacetyl bromide, complicated operation, easy hydrolysis of bromoacetyl bromide, etc., and achieves the effect of improving silanization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
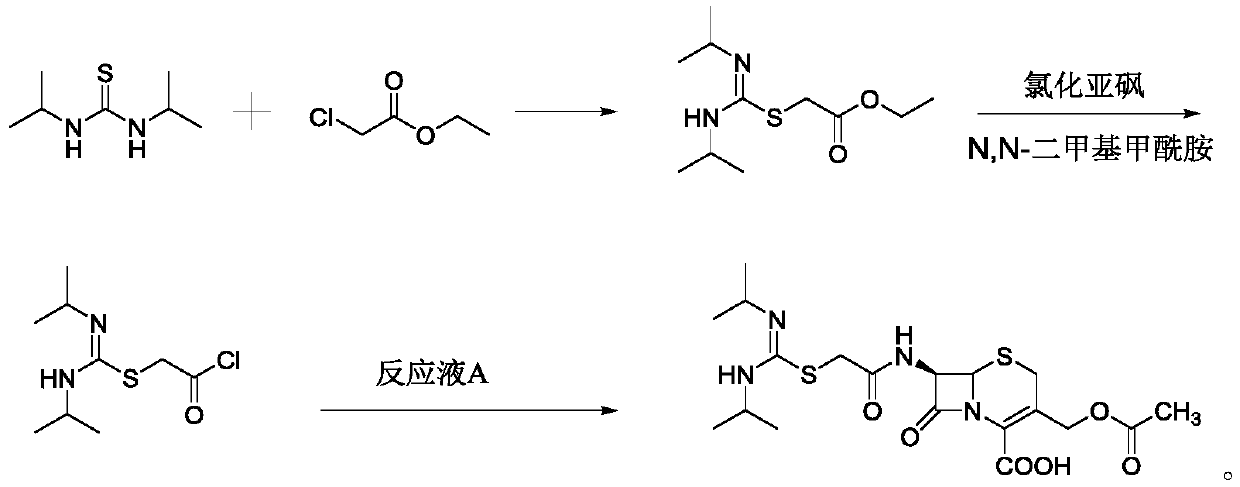
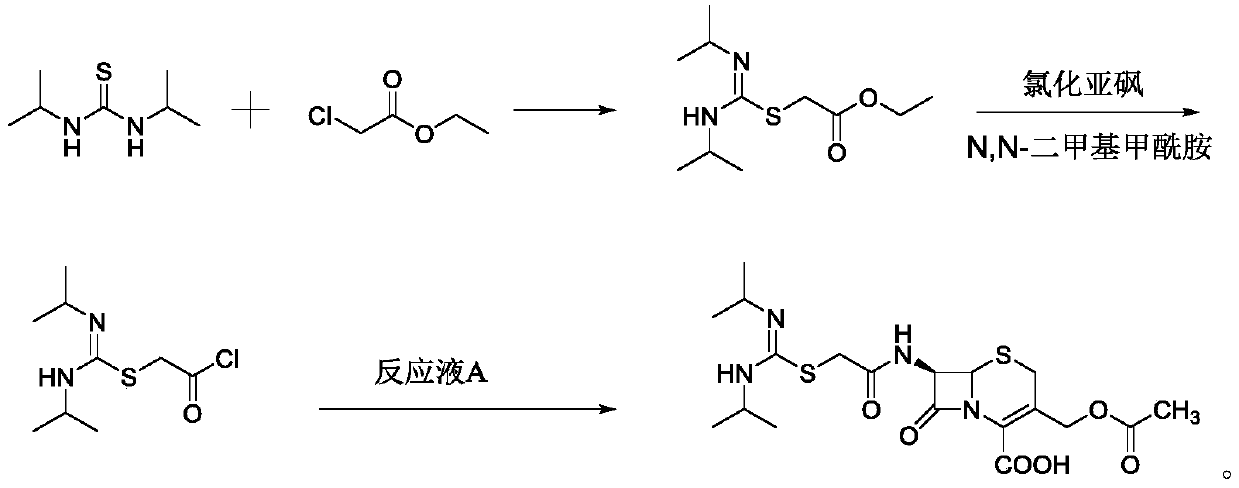
[0030] Add 60mL of dichloromethane into a 250mL reaction bottle, add 10.88g of 7-ACA while stirring, add 7.10g of hexamethyldisilazane, 1.74g of trimethylchlorosilane, stir at room temperature for 1h, and monitor the reaction by HPLC to form Reaction solution A is reserved.
[0031] Add 60mL of dichloromethane to a 500mL reaction bottle, add 6.40g of N,N-diisopropylthiourea, add 5.09g of sodium carbonate, 0.64g of sodium iodide, control the temperature at 15-25°C, add 1.80g of ethyl chloroacetate, Stir for 15 minutes, add 1.80 g of ethyl chloroacetate, stir for 15 minutes, add 1.80 g of ethyl chloroacetate, stir at a temperature of 20 to 25°C for 2 hours, HPLC detects that the reaction is complete, add concentrated hydrochloric acid to adjust the pH to 1.5, stir for 30 minutes, and cool down to - 5-5°C, add 5.23g of thionyl chloride, slowly dropwise add 2.92g of N,N-dimethylformamide, stir at -5-5°C for 1h, HPLC detects that the reaction is over, add reaction solution A, add N...
Embodiment 2
[0033] Add 60mL of dichloromethane into a 250mL reaction bottle, add 10.88g of 7-ACA while stirring, add 7.75g of hexamethyldisilazane, 2.17g of trimethylchlorosilane, stir at room temperature for 1h, and monitor the reaction by HPLC to form Reaction solution A is reserved.
[0034] Add 60mL of dichloromethane to a 500mL reaction bottle, add 6.40g of N,N-diisopropylthiourea, add 7.19g of potassium carbonate, 0.64g of potassium iodide, control the temperature at 15-25°C, add 1.80g of ethyl chloroacetate, and stir for 15min , add 1.80 g of ethyl chloroacetate, stir for 15 min, add 1.80 g of ethyl chloroacetate, stir at 20-25°C for 2 h, HPLC detects that the reaction is over, add hydrochloric acid to adjust the pH to 2.0, stir for 30 min, and cool down to -5-5 ℃, add 5.23g of thionyl chloride, slowly add 2.92g of N,N-dimethylformamide dropwise, control the temperature at -5~5℃ and stir for 1h, HPLC detects that the reaction is over, add reaction solution A, add N,N- 5.23g of dim...
Embodiment 3
[0036] Add 60mL of dichloromethane into a 250mL reaction bottle, add 10.88g of 7-ACA while stirring, add 8.39g of hexamethyldisilazane, 2.61g of trimethylchlorosilane, stir at room temperature for 1h, monitor the reaction by HPLC, and form Reaction solution A is reserved.
[0037] Add 60mL of dichloromethane to a 500mL reaction bottle, add 6.40g of N,N-diisopropylthiourea, add 4.70g of sodium bicarbonate, 0.64g of sodium iodide, control the temperature at 15-25°C, and add 1.80g of ethyl chloroacetate , stirred for 15 minutes, added 1.80 g of ethyl chloroacetate, stirred for 15 minutes, added 1.80 g of ethyl chloroacetate, stirred at a temperature of 20-25°C for 2 hours, HPLC detected that the reaction was completed, added hydrochloric acid to adjust the pH to 2.5, stirred for 30 minutes, and cooled to - 5-5°C, add 5.23g of thionyl chloride, slowly dropwise add 2.92g of N,N-dimethylformamide, stir at -5-5°C for 1h, HPLC detects that the reaction is over, add reaction solution A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com