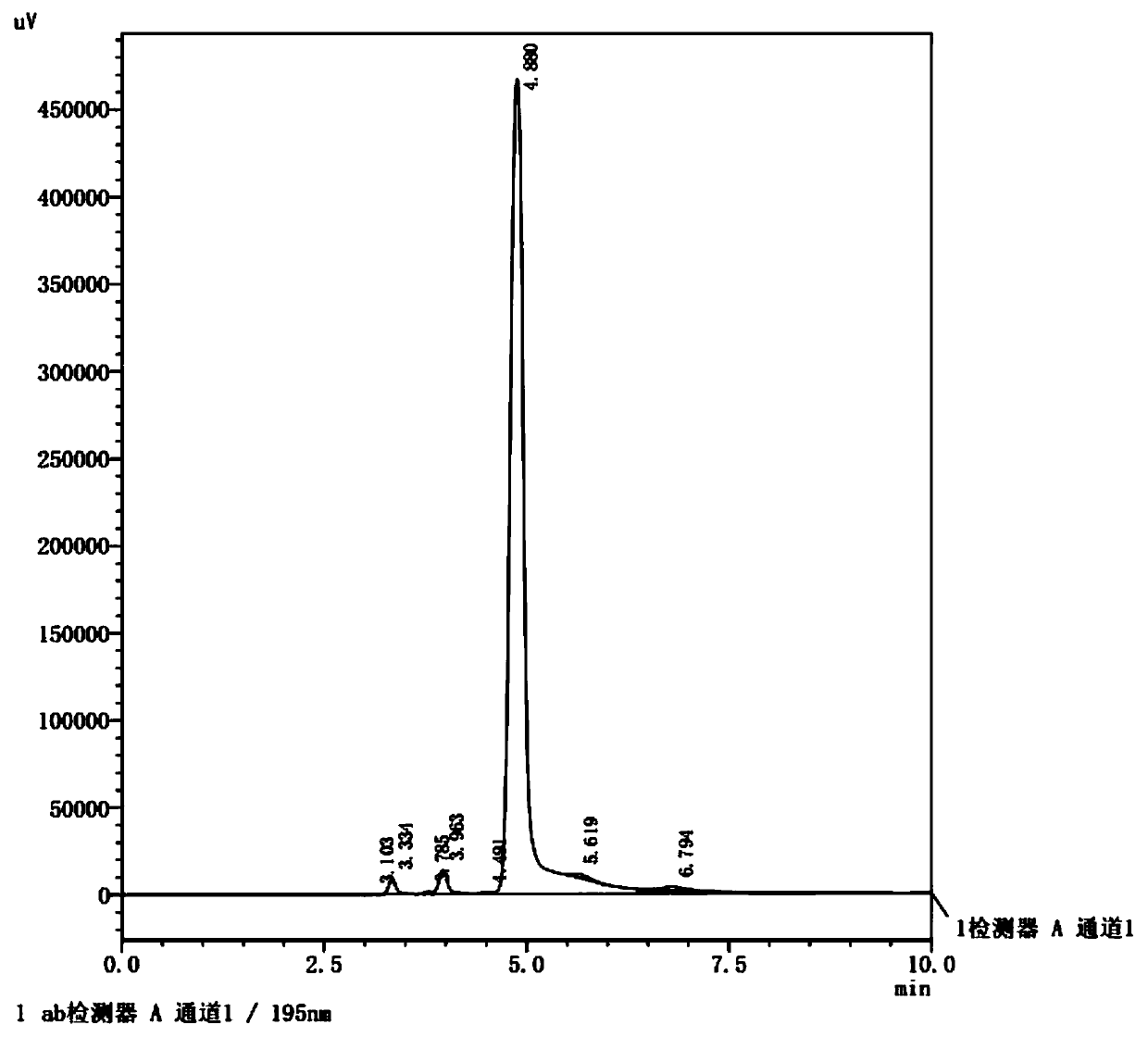
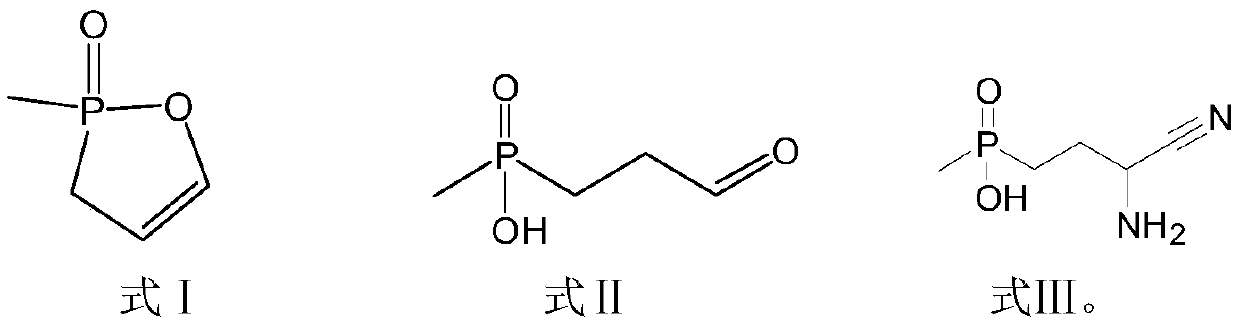
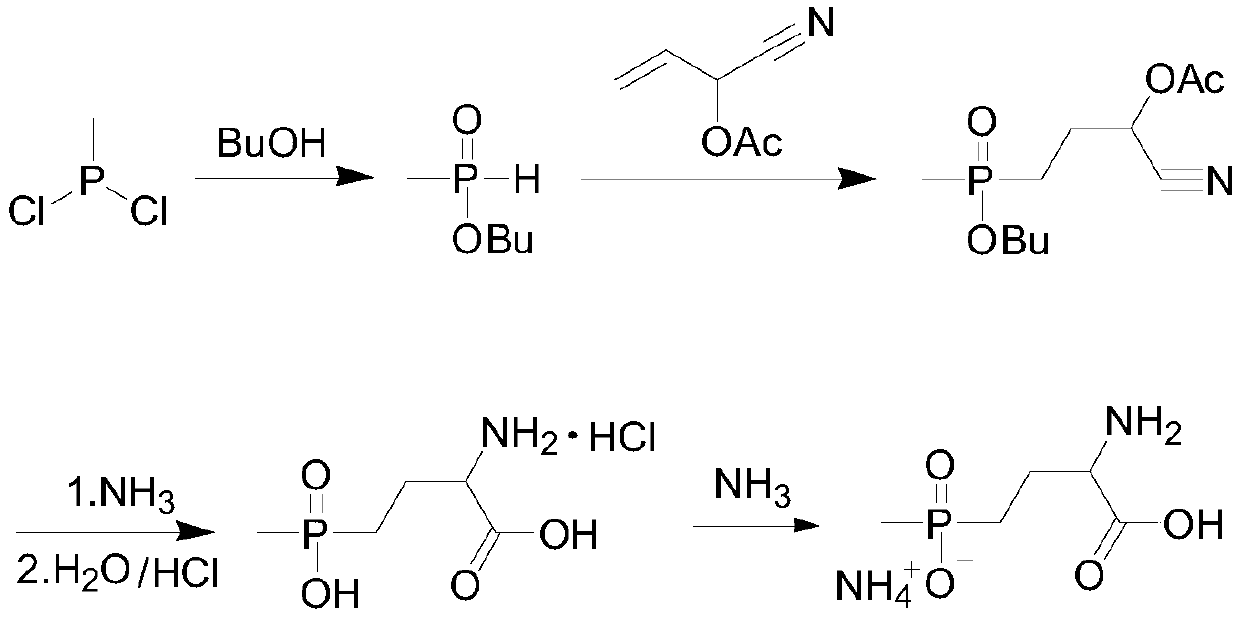
Preparation method of glufosinate-ammonium
A technology of glufosinate-ammonium and phosphonic-aldehyde, which is applied in the field of pesticide chemistry, can solve problems such as few examples and no research reports on glufosinate-ammonium, and achieve the effects of avoiding waste water, good environmental friendliness, and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 12.0 g of methyl phosphine dichloride dropwise to the mixed solution of acrolein (6.2 g) and acetic anhydride (11.5 g), and maintain the reaction at -5 to 0 ° C. After the dropwise addition, maintain the temperature and continue the reaction for 1 h. Then place the reaction solution at 20-30°C for 2 hours to end the reaction; use a rotary evaporator (temperature 50-70°C, vacuum degree above -0.09MPpa) to remove the low-boiling by-product acetyl chloride After removing unreacted acrolein and acetic anhydride, add 5.1g of water, fully stir and hydrolyze at a temperature of 20-40°C for 1h; add sodium cyanide (5.5g) and ammonium chloride (11.8g) dropwise to the obtained hydrolyzate , ammonia water (45mL, 20%), stirred and reacted at 20-40°C for 4h, and the excess ammonia water was removed by rotary evaporation to obtain the intermediate aminonitrile solution; the obtained aminonitrile solution was refluxed with 300mL hydrochloric acid (35%) for hydrolysis After 4 hours,...
Embodiment 2
[0041] Add 24.2g of methyl phosphine dichloride dropwise to the mixed solution of acrolein (12.9g) and acetic anhydride (24.5g), maintain the reaction at -5~0°C, after the dropwise addition, keep the temperature and continue the reaction for 1.5h , then place the reaction solution at 20-30°C for 2.5h to end the reaction; use a rotary evaporator (temperature 50-70°C, vacuum degree above -0.09MPpa) to remove low-boiling by-products After acetyl chloride, unreacted acrolein and acetic anhydride, add 10.2g of water, fully stir and hydrolyze at 20-40°C for 1 hour; add sodium cyanide (11.1g), ammonium chloride (23.9 g), a mixed solution of ammonia water (90mL, 20%), stirred and reacted at 20-40°C for 4h, and the excess ammonia water was removed by rotary evaporation to obtain the intermediate aminonitrile solution; the aminonitrile solution was hydrolyzed with 500mL hydrochloric acid (35%) under reflux After 4 hours, the excess hydrochloric acid was removed by rotary evaporation, th...
Embodiment 3
[0043] Add 36.1g of methyl phosphine dichloride dropwise to the mixed solution of acrolein (19.1g) and acetic anhydride (34.6g), maintain the reaction at -5~0°C, after the dropwise addition, keep the temperature and continue the reaction for 2h, Then put the reaction solution at 20-30°C for 3h to end the reaction; use a rotary evaporator (temperature 50-70°C, vacuum above -0.09MPpa) to remove the low-boiling by-product B After acid chloride, unreacted acrolein and acetic anhydride, add 16.1g of water, and fully stir and hydrolyze at a temperature of 20-40°C for 1.5h; add sodium cyanide (16.5g), ammonium chloride (35.9 g), a mixed solution of ammonia water (140mL, 20%), stirred and reacted at 20-40°C for 4h, and the excess ammonia water was removed by rotary evaporation to obtain the intermediate aminonitrile solution; the aminonitrile solution was hydrolyzed with 800mL hydrochloric acid (35%) under reflux After 4 hours, the excess hydrochloric acid was removed by rotary evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com