Application of aluminoborate as fluorine adsorbent
A boro-aluminate and adsorbent technology, applied in the directions of borate, adsorbed water/sewage treatment, boron compounds, etc., can solve the problems of high cost and unusable adsorbent, and achieve low cost, strong adsorption, and suitable wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] In the present invention, the preparation method of the boroaluminate preferably comprises the following steps:
[0021] (1) Boric acid, aluminum chloride, ammonia water and water are mixed under heating conditions, then cooled to room temperature, and hydrochloric acid is used to adjust the pH value of the mixed solution to 3.0-3.5 to obtain a gel;
[0022] The mass ratio of the boric acid and aluminum chloride is 25-35:15-25;
[0023] (2) subjecting the gel to hydrothermal crystallization to obtain the boroaluminate.
[0024] In the invention, boric acid, aluminum chloride, ammonia water and water are mixed under heating conditions, then cooled to room temperature, and hydrochloric acid is used to adjust the pH value of the mixed solution to 3.0-3.5 to obtain a gel. In the present invention, the mass ratio of boric acid to aluminum chloride is preferably 29:18. In the present invention, the water is preferably deionized water; the mass ratio of boric acid to water i...
Embodiment 1
[0038] (1) 15g of boric acid was added to 120g of deionized water, and dissolved in a 70°C water bath to obtain a clear solution;
[0039] (2) Add 9 g of aluminum chloride to the solution obtained in step (1), stir for 30 minutes, then add 12 mL of aqueous ammonia with a mass concentration of 28%, stir for 1.5 hours, and cool to room temperature;
[0040] (3) Using hydrochloric acid with a concentration of 12mol / L to adjust the pH value of the solution obtained in step (2) to 3.1 to obtain a gel;
[0041] (4) Put the gel obtained in step (3) into a reaction kettle, put it in a 160°C oven for hydrothermal crystallization for 1 day; cool the crystallized product to room temperature, centrifuge the crystallized product, and Wash with distilled water and dry to obtain boroaluminate.
[0042] Characterize the boroaluminate obtained in Example 1, and test the adsorption effect on fluoride ions, specifically as follows:
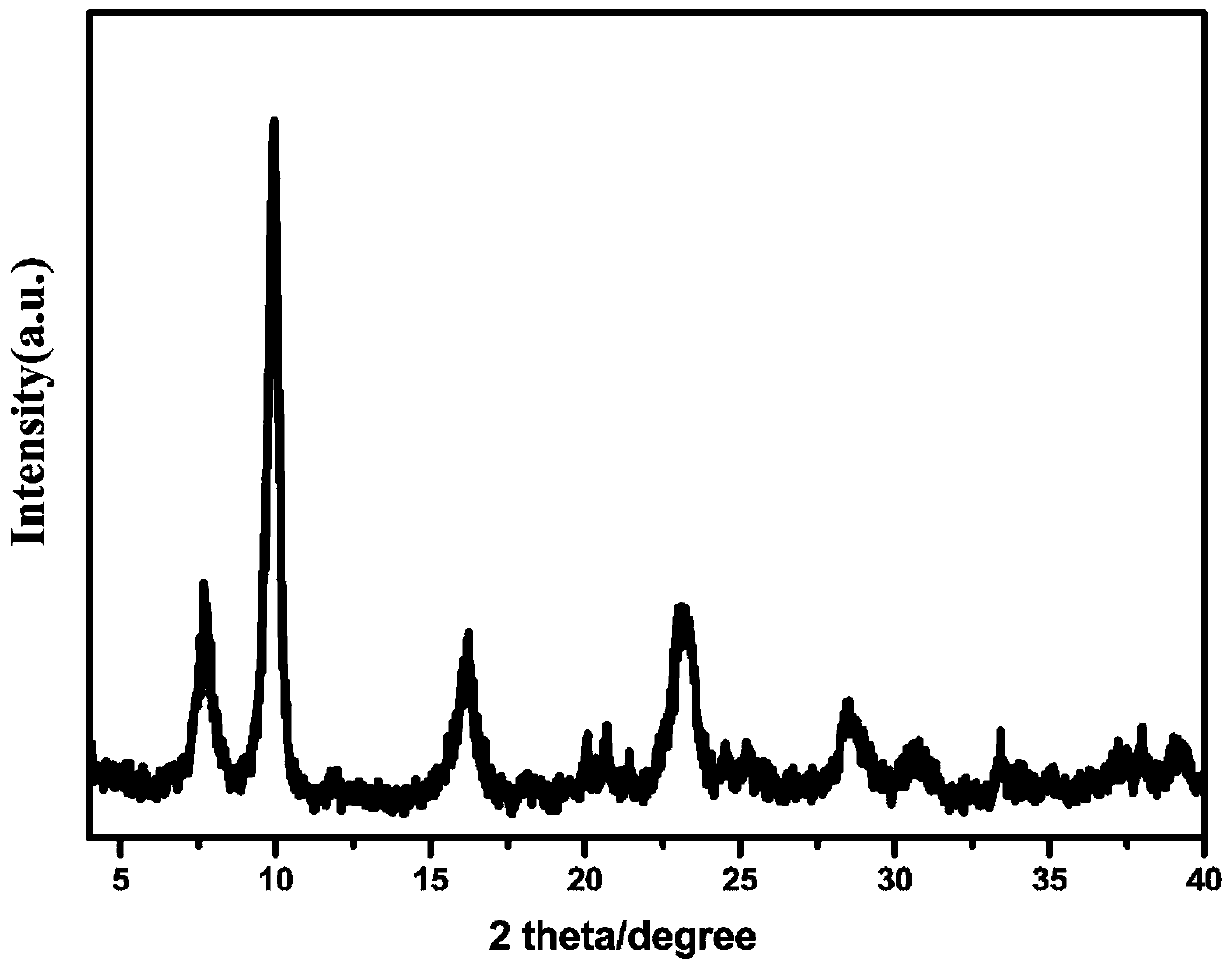
[0043] (1) Characterize the boroaluminate obtained in Example ...
Embodiment 2
[0063] Change the temperature of hydrothermal crystallization in step (4) of Example 1 to 150°C, and the others are the same as in Example 1 to obtain boroaluminate.
[0064] The X-ray powder diffraction pattern and the scanning electron microscope image of the boroaluminate that embodiment 2 obtains are respectively with figure 1 , figure 2 Similarly, the boroaluminate obtained in Example 2 is a cationic framework material with a lamellar morphology.
[0065] Adopt the method for above-mentioned (A) process to test the adsorption capacity of the boroaluminate that embodiment 2 obtains, record to F - The adsorption rate is 95.7%, and the adsorption capacity is 38.28mg / g.
[0066] Adopt the method of above-mentioned (B) process to test other competing ions (Cl - , NO 3 - ) to the influence of embodiment 2 boroaluminate adsorption fluoride ions, even if there is a certain amount of negative monovalent anion in the result, the boroaluminate that embodiment 2 obtains is to F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com