Preparation and application of oligomer photovoltaic donor material based on 5,6-difluorobenzothiadiazole unit
A photovoltaic material and oligomer technology, which is applied in the synthesis and application field of A'2DA' type oligomer donor material, can solve the problem that the relationship between photovoltaic performance is not clear, and achieves high yield, wide light absorption range, high The effect of open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis route of A'-D-A-D-A' type organic small molecule donor material SM1 is as follows:
[0055]
[0056] 1.1 Synthesis of compound 3
[0057] Under nitrogen protection, compound 1 (6.0 g, 12.40 mmol), compound 2 (1.63 g, 4.94 mmol), Pd(PPh 3 ) 4 (0.29 g, 5% mol) and 48 mL toluene, 110 o C was refluxed for 3 h, stopped the reaction, extracted with dichloromethane, combined the organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered with suction, and spin-dried the solvent. The crude product was purified by column chromatography using petroleum ether as the eluent. 2.38 g of orange-yellow solid compound 3 was isolated with a yield of 86%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.09 (s, 2H), 7.19 (s, 2H), 2.66 (d, J = 6.9 Hz, 4H), 1.65-1.60 (m,2H), 1.40 - 1.25 (m, 16H), 0.94-0.90 (m, 12H).
[0058] 1.2 Synthesis of compound 4
[0059] In a 100 mL single-necked flask, compound 3 (2.3 g, 4.10 mmol), N-bromosuccinimide (1.68 g,...
Embodiment 2
[0067] A'(D-A) 2 The synthetic route of DA'-type oligomer donor material SM2 is as follows:
[0068]
[0069] 2.1 Synthesis of Compound 7
[0070] Under nitrogen protection, (5-(1,3-dioxolan-2-yl)thiophen-2-yl)tributyltin (2.48 g, 5.57 mmol), compound 4 (4.0 g, 5.57 mmol), Pd (PPh 3 ) 4 (0.32 g, 5% mol) and 70 mL toluene, 110 o C was refluxed for 12 h, stopped the reaction, cooled to room temperature, and distilled off the toluene solvent under reduced pressure. The remaining crude product was dissolved in 35 mL of tetrahydrofuran, added aqueous hydrochloric acid (10%, 20 mL), and stirred overnight at room temperature. Transfer the reaction solution to a separatory funnel, extract with dichloromethane, combine the organic phases, wash with saturated brine, dry over anhydrous magnesium sulfate, filter with suction, and spin to dry the solvent. The crude product is separated into petroleum ether: dichloromethane ( V: V , 1: 1) as the eluent, separated by column chromat...
Embodiment 3
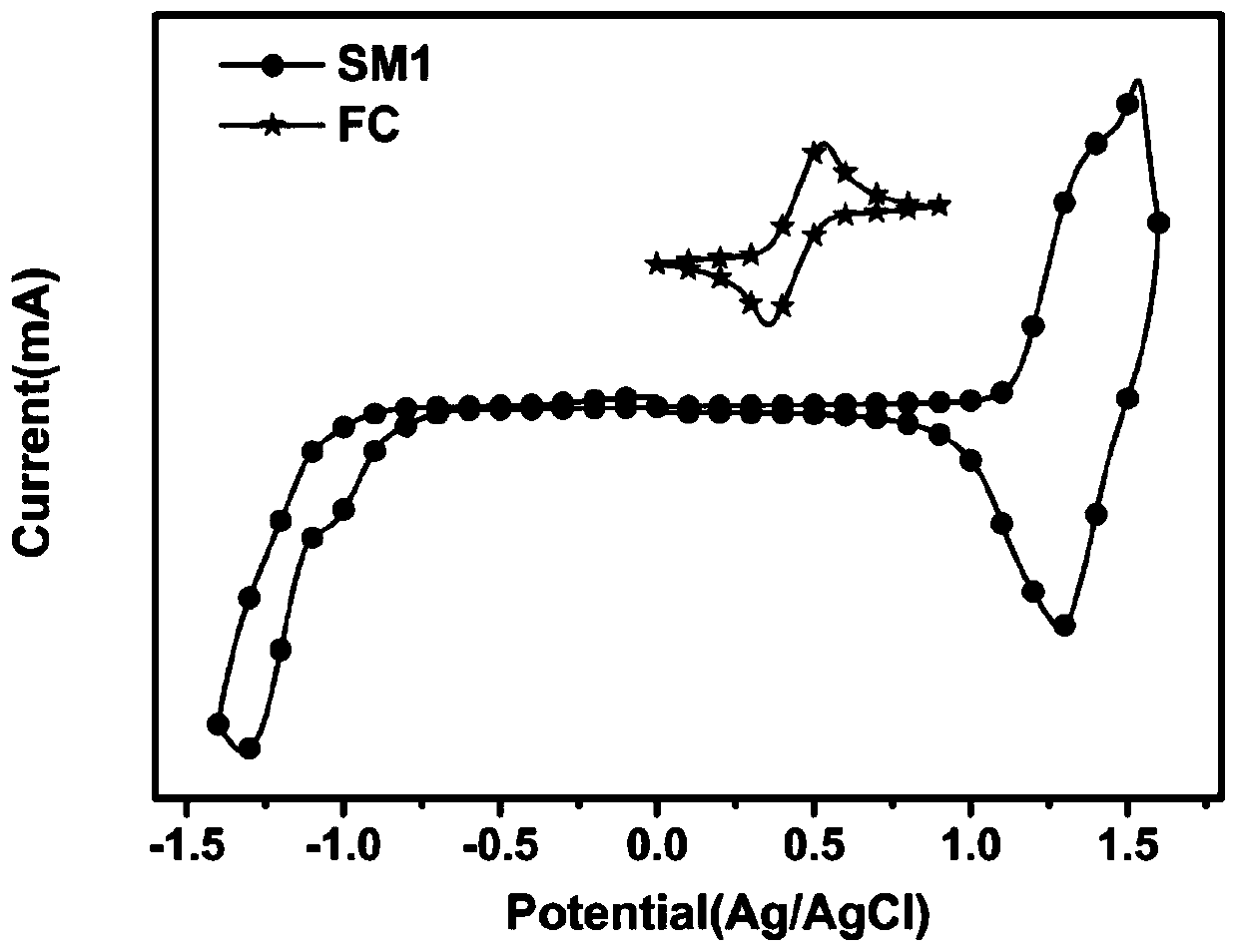
[0076] Photophysical, electrochemical and thermodynamic stability tests of the small molecule SM1 synthesized in Example 1.
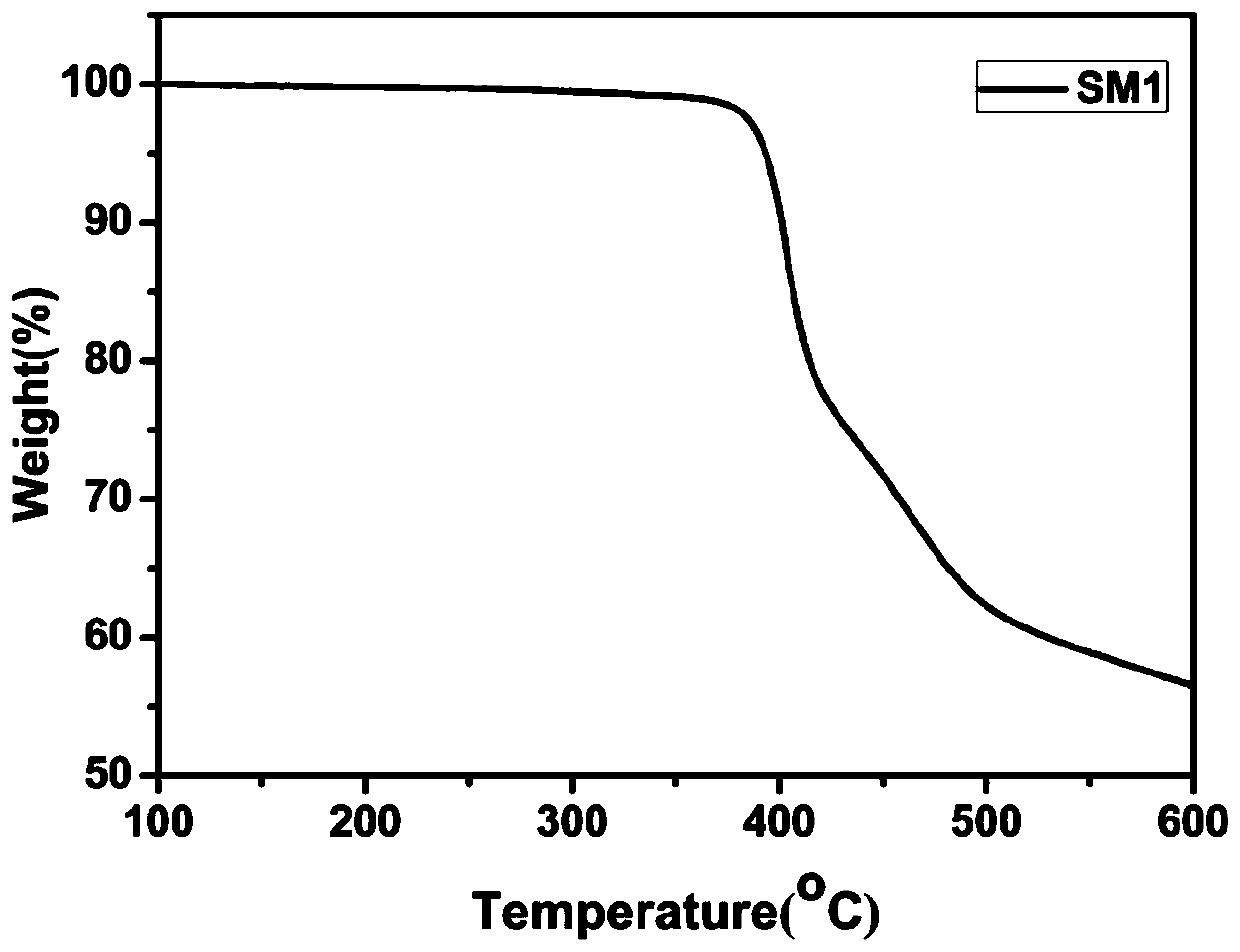
[0077] The thermogravimetric curve of small molecule SM1 is as follows: figure 1 As shown, its decomposition temperature is 393 o c.
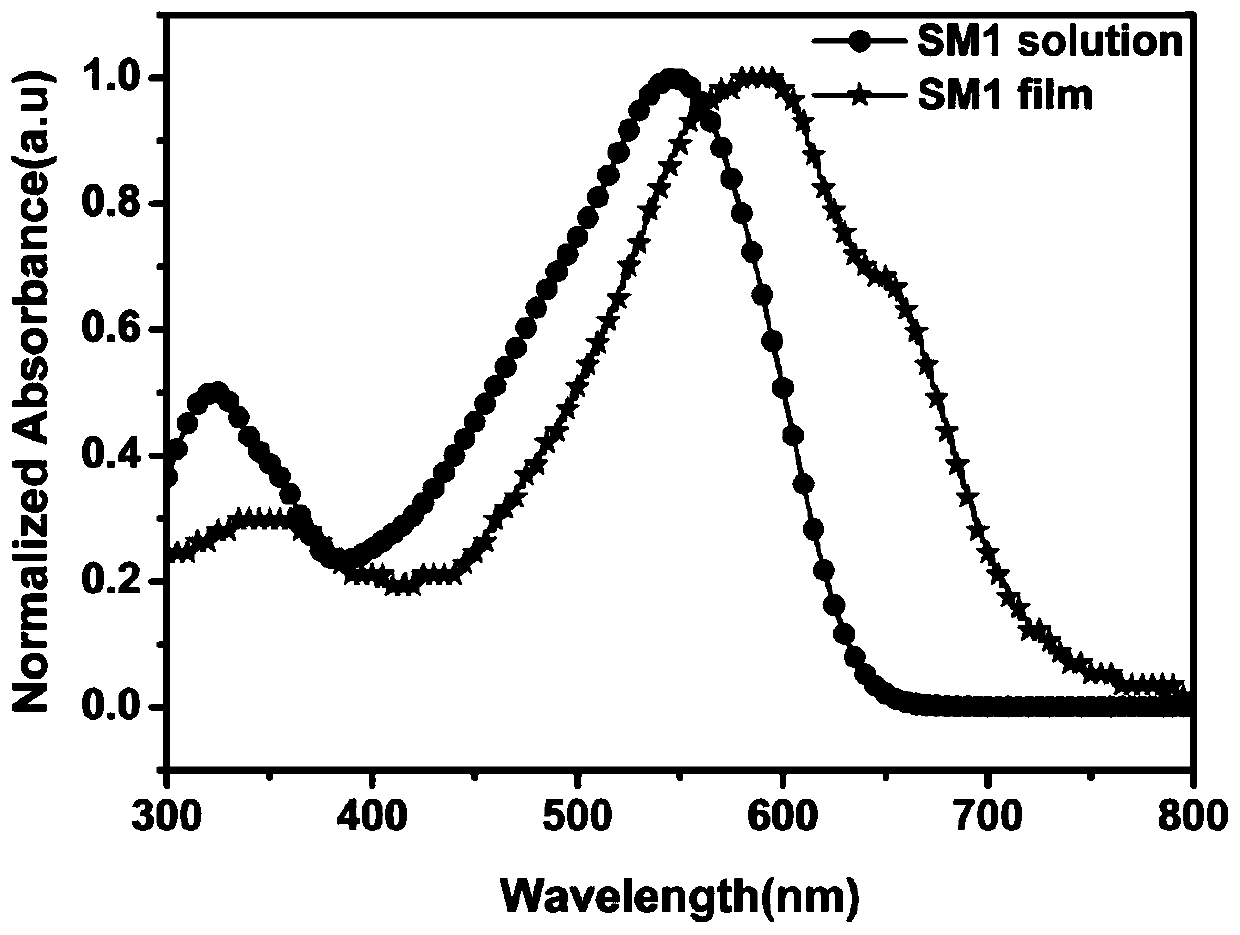
[0078] The UV-Vis absorption spectra of small molecule SM1 in chloroform solution and solid film such as figure 2 shown. Exhibits strong absorption at 300-650 nm. The absorption peak around 330 nm is the π-π* transition absorption peak of the molecule, and the absorption peak at 547 nm can be attributed to the charge transfer from the donor unit thiophene to the acceptor unit benzothiadiazole and terminal cyano-rhodanine (ICT ) transition absorption peak. Compared with the absorption spectrum of the solution, the absorption peak of the solid film of small molecule SM1 has a red shift and an absorption shoulder peak, which is caused by the accumulation of molecules in the solid film. The measured peak position is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com