Method for preparing hexamethylenediamine from adipic dialdehyde in fixed bed reactor
A fixed-bed reactor and adipaldehyde technology, applied in chemical instruments and methods, reduction alkylation preparation, molecular sieve catalysts, etc., can solve the problems of high toxicity of adiponitrile, dependence on imports, high price, etc., and achieve improved selection Performance and catalyst stability, route cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 metal@molecular sieve catalyst
[0071] (1) 2.48gNi(NO 3 ) 2 ·6H 2 O was dissolved in water, and the volume was adjusted to 12mL, and 10g of HBeta molecular sieves (SiO 2 / Al 2 o 3 Molar ratio = 800, SiO 2 The mass content is 98.9%), the Ni element is loaded on the HBeta by the equal volume impregnation method, placed in a 100 ° C oven for 12 h, and then roasted in a 500 ° C muffle furnace for 4 h, H 2 Reduction at 500°C for 4 hours in the atmosphere to obtain metal-loaded Ni / HBeta molecular sieves;
[0072] (2) Place metal-loaded Ni / HBeta molecular sieves in 15 mL of TPAOH aqueous solution with a mass fraction of 35.0%, immerse at room temperature for 2 h, and dry in an oven at 100° C. for 12 h to obtain TPAOH / HBeta molecular sieves adsorbed in the pores;
[0073] (3) Mix the / HBeta molecular sieve with TPAOH adsorbed in the channel with 0.60g sodium hydroxide and 20mL deionized water, and crystallize at 90°C for 6 hours to obtain...
Embodiment 2-10
[0076] The preparation of embodiment 2-10 metal@molecular sieve catalyst
[0077] The same steps in Example 1 were used to prepare M@ZSM-5, and the metal type and loading amount were adjusted by changing the type and concentration of the metal salt solution. The specific synthesis conditions are listed in Table 1. The mass content of metals in M@ZSM-5 was tested by ICP-OES.
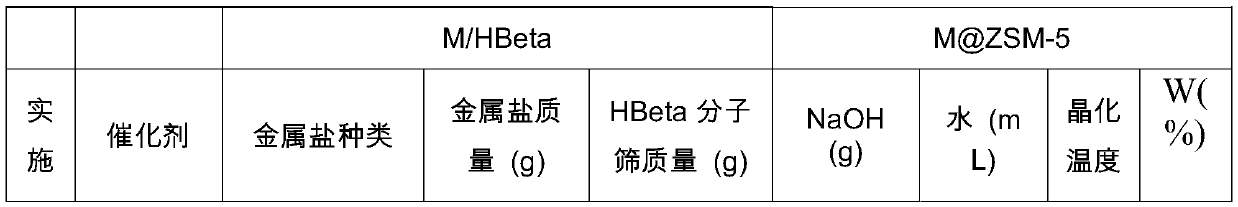
[0078] The specific synthetic conditions of each embodiment of table 1
[0079]
[0080]
[0081] In the table, in the catalyst nA@ZSM-5, A represents the transition metal, ZSM-5 is the abbreviation of HZSM-5, and n represents the mass loading of the noble metal in the M / HBeta molecular sieve. W(%) represents the mass content of metal in M@ZSM-5.
[0082] Embodiment 1-10 Characterization of metal@molecular sieve catalyst
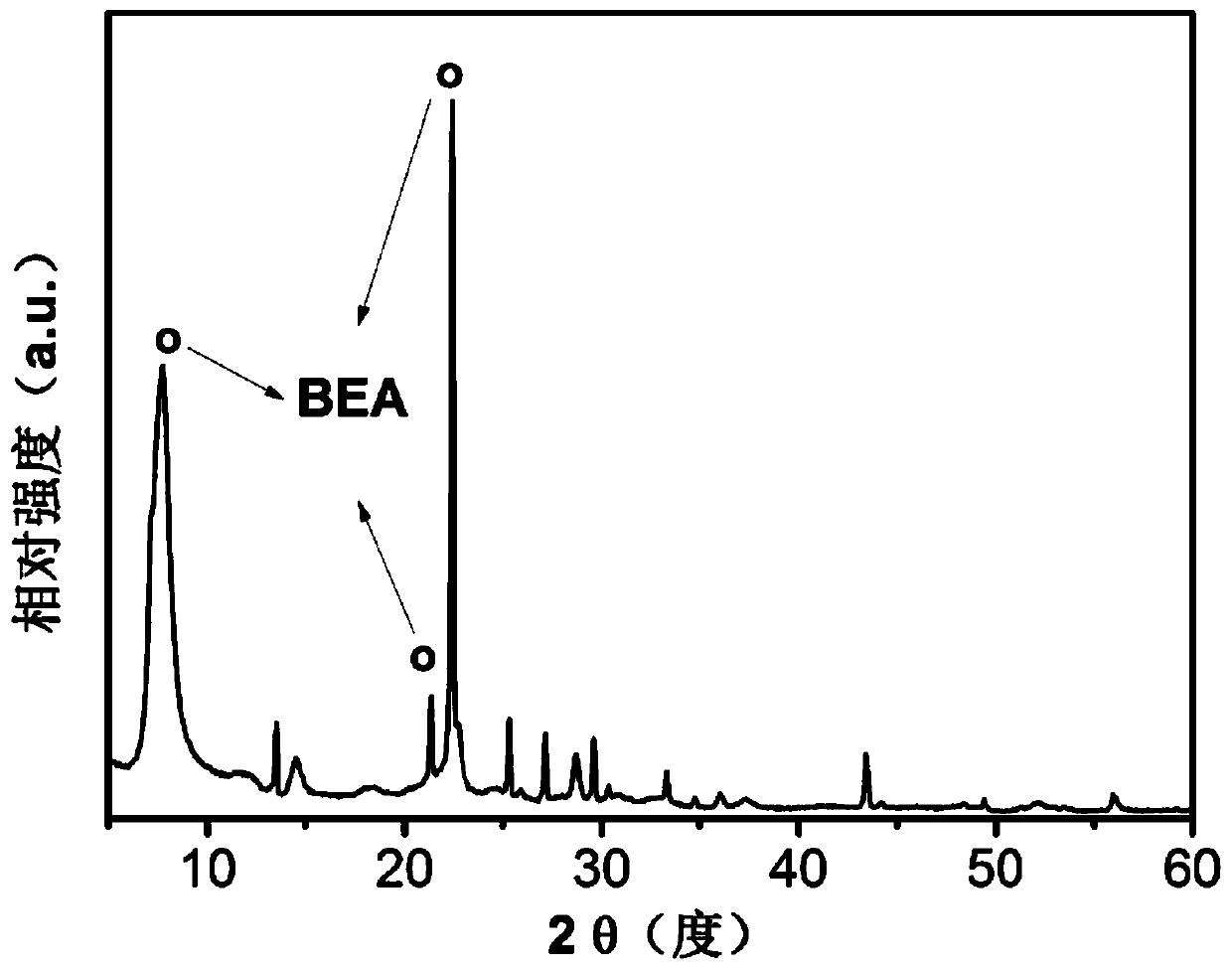
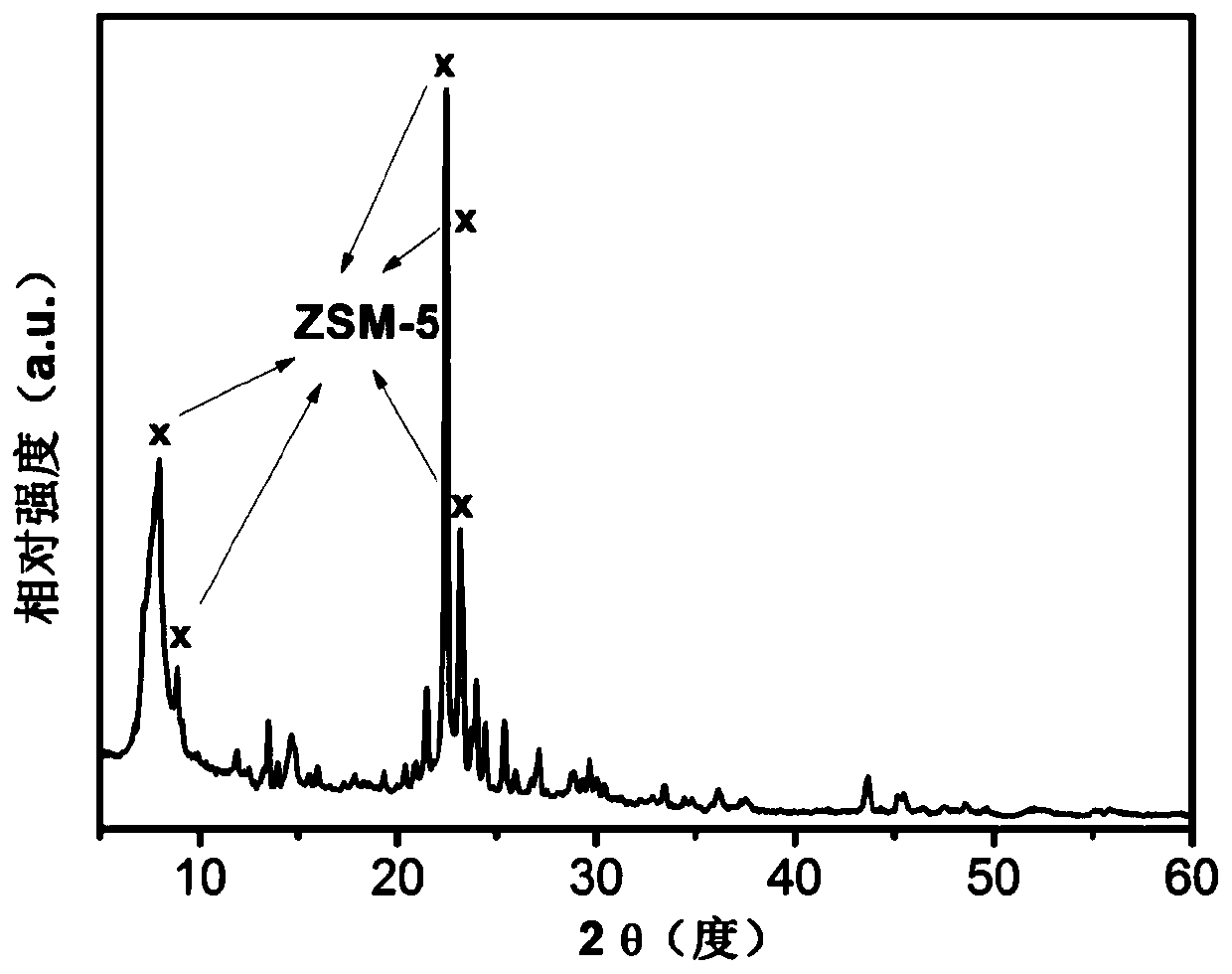
[0083] figure 1 It is the XRD spectrogram of the HBeta molecular sieve used in Example 1; the catalyst obtained in Examples 1 to 10 is a ZSM-5 molecular sieve, and a typical r...
Embodiment 11-20
[0084] Reaction Performance Evaluation of Embodiment 11-20 Metal @ Molecular Sieve Catalyst
[0085] Pack 2.0 g of the above-mentioned catalyst in a small fixed-bed reactor, which is a stainless steel reaction tube with an inner diameter of 10 mm and a length of 300 mm. Both ends of the catalyst are filled with quartz sand. gas, the catalyst was subjected to reduction treatment at 400°C for 4 hours, wherein the reducing gas composition was H 2 / N 2 The volume ratio is 1 / 4.
[0086] After the reduction, the temperature of the reactor dropped to 130°C, the reaction pressure rose to 6.0Mpa, and H 2 , liquid ammonia and adipaldehyde for reductive amination reaction, wherein liquid ammonia and adipaldehyde are respectively injected into the reaction system through a high-pressure micro-feed pump, and the mass space velocity of adipaldehyde is 1.0h -1 , H 2 :NH 3 : adipaldehyde=15:30:1, reaction 10h sampling adopts gas chromatography analysis, and reaction result is listed in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com