Preparation method of cyclic methylene disulfonate compound
A technology of methylene disulfonate and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of low product purity, low yield, poor product color, etc., and achieve the effect of simple post-processing and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
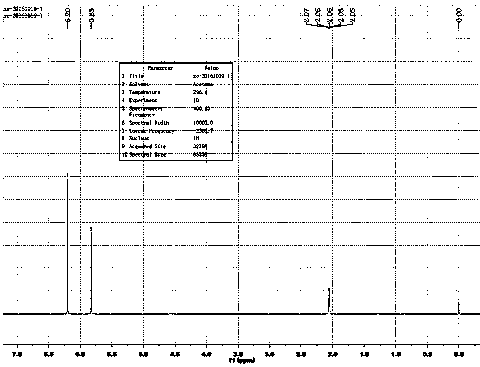
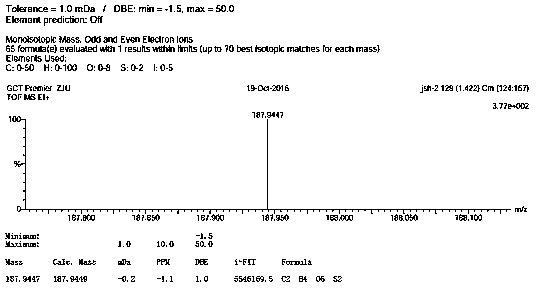
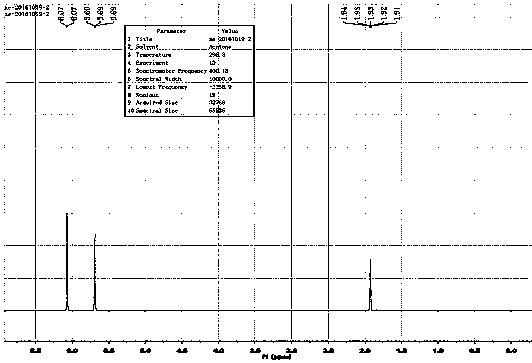
Image
Examples
Embodiment 1
[0043] In a stainless steel autoclave with a volume of 250 milliliters, add 42.28 grams of methanedisulfonic acid (240 mmoles), phosphorus pentoxide (400 mmoles), methylal (480 mmoles), 2 grams of preferred scheme one Prepare the iron-molybdenum-bismuth composite oxide supported on silica gel, then close the reaction kettle, completely replace the air in the reaction kettle with oxygen, then feed oxygen, keep the pressure at 1.8-2 kg, heat up to 200°C under stirring, keep stirring Reaction for 12 hours, the pressure of oxygen supplemented intermittently. After the reaction is over, cool the reaction solution, evaporate and recover unreacted methylal, stir the residue with 500 milliliters of ethyl acetate and 200 milliliters of cold water, then suction filter, wash the filter cake with ethyl acetate and cold water successively (filter cake The iron-molybdenum-bismuth composite oxide loaded on silica gel obtained by drying at 140 degrees for 4 hours, the quality is 2 grams), the...
Embodiment 2
[0046] In a stainless steel autoclave with a volume of 250 milliliters, add 42.28 grams of methanedisulfonic acid (240 millimoles), phosphorus pentoxide (350 millimoles), methylal (240 millimoles), 3 grams of Example 1 Prepared iron-molybdenum-bismuth composite oxide supported on silica gel, then closed the reaction kettle, completely replaced the air in the reaction kettle with oxygen, then introduced oxygen, kept the pressure at 2 kg, raised the temperature to 180°C under stirring, and kept stirring for 15 Hours, the pressure of intermittent supplementary oxygen. After the reaction, cool the reaction liquid, evaporate and recover unreacted methylal, stir the residue with 500 ml of ethyl acetate and 200 ml of cold water, then suction filter, wash the filter cake with ethyl acetate and cold water successively, and separate the filtrate The upper organic phase was taken out, decolorized with activated clay, and then concentrated to obtain the crude product, which was recrystall...
Embodiment 3
[0048] In a stainless steel autoclave with a capacity of 250 milliliters, add 42.28 grams of methanedisulfonic acid (240 millimoles), phosphorus pentoxide (420 millimoles), methylal (900 millimoles) respectively, 3 grams of Example 1 The prepared silica gel-loaded iron-molybdenum-bismuth composite oxide was loaded in a quartz tube (diameter 1cm, length 3cm) that was not closed at both ends, and the quartz tube was fixed above the liquid level of the reactor with a steel bracket, and then the reactor was sealed. Completely replace the air in the reactor with oxygen, then introduce oxygen, keep the pressure at 2 kg, raise the temperature to 230°C under stirring, keep warm and stir for 24 hours, and supplement the pressure of oxygen intermittently. After the reaction, cool the reaction liquid, evaporate and recover unreacted methylal, stir the residue with 500 ml of ethyl acetate and 200 ml of cold water, then suction filter, wash the filter cake with ethyl acetate and cold water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com