Polymeric microsphere with disaccharide-based skeleton and preparation method of polymeric microsphere
A technology of polymerizing microspheres and disaccharide groups, which is applied in the preparation of microspheres, microcapsule preparations, surgical adhesives, etc., can solve the problems of high swelling and poor biocompatibility of microspheres, and achieve good sphericity and smooth surface , The effect of simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 5 g of maltose (equivalent to 1 part of maltose) into a three-neck flask filled with 25 ml of pure water, stir and disperse evenly. Then add 5.302g of bromopropene (equivalent to 3 parts of bromopropene), adjust the pH to 11, and after magnetic stirring for 15min, 0.050g of tetrabutylammonium perchlorate (equivalent to 0.01 part of tetrabutylammonium perchlorate) Add to the reaction solution. The mixed solution was heated to 75° C., and the reaction was continued to stir for 24 hours. After extraction, it was washed and dried, and purified by chromatographic column to obtain allyl digose ether.
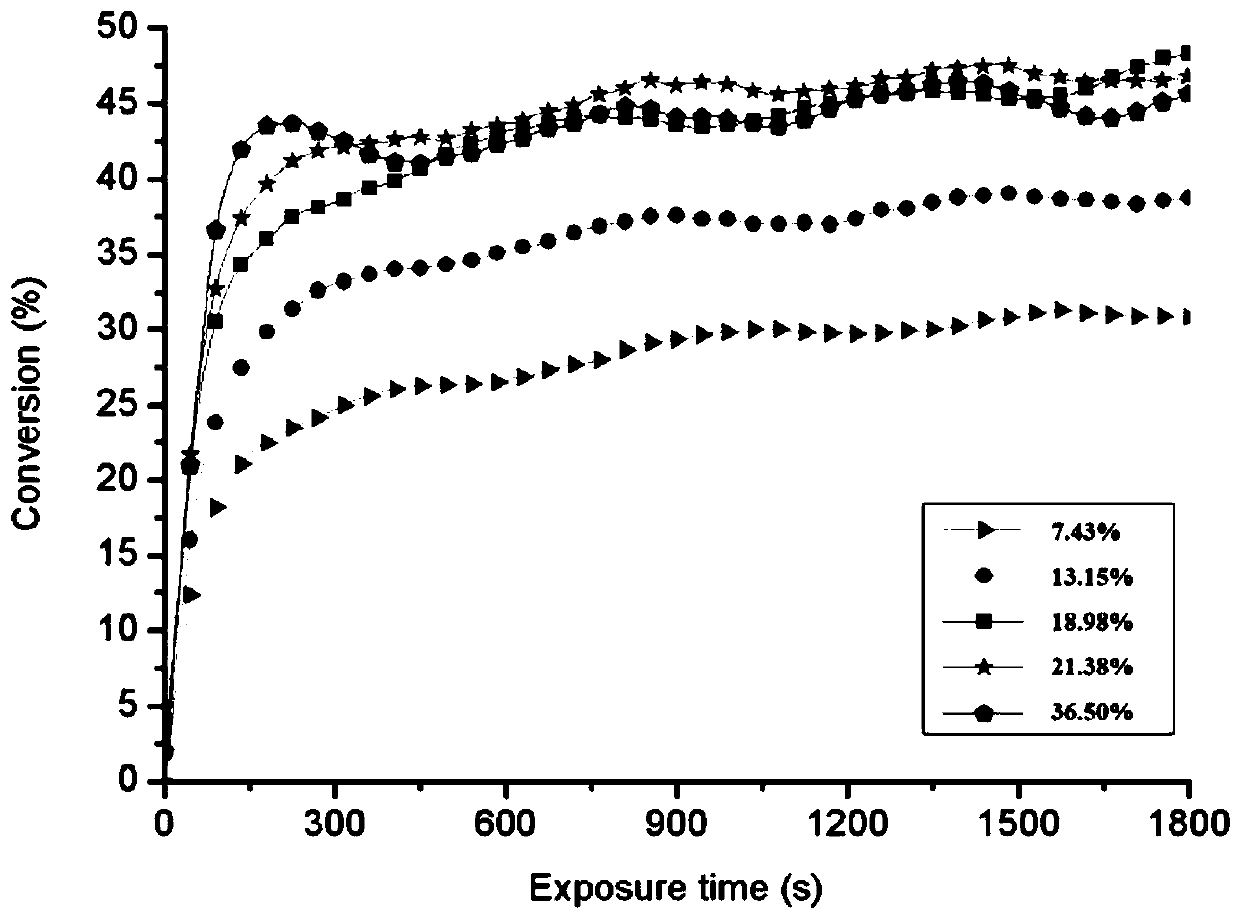
[0047] Prepare the molar ratio of photoinitiator HMPP and allyl disaccharide ether respectively as 7.43%, 13.15%, 18.98%, 21.38% and 36.50% of the mixture, evenly spread on the dried potassium bromide sheet, use Fourier transform Kinetic curves of photopolymerization were obtained by real-time infrared (RT-IR).
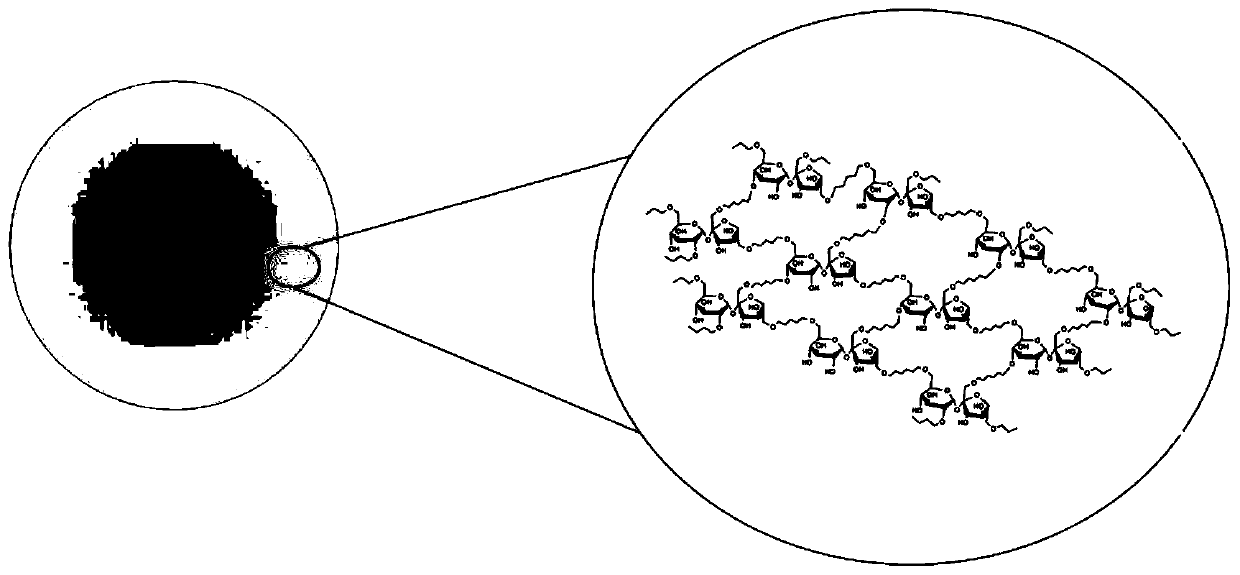
[0048] figure 1 is the cross-linked structure of microsphe...
Embodiment 2
[0050] Add 5 g of sucrose (equivalent to 1 part of sucrose) into a three-neck flask filled with 15 ml of pure water, stir and disperse evenly. Then add 3.535g of bromopropene (equivalent to 2 parts of bromopropene), adjust the pH to 9, and after magnetic stirring for 15min, mix 0.050g of tetrabutylammonium perchlorate (equivalent to 0.01 part of tetrabutylammonium perchlorate) Add to the reaction solution. The mixed solution was heated to 75° C., and the reaction was continued to stir for 24 hours. After extraction, it was washed and dried, and purified by chromatographic column to obtain allyl digose ether.
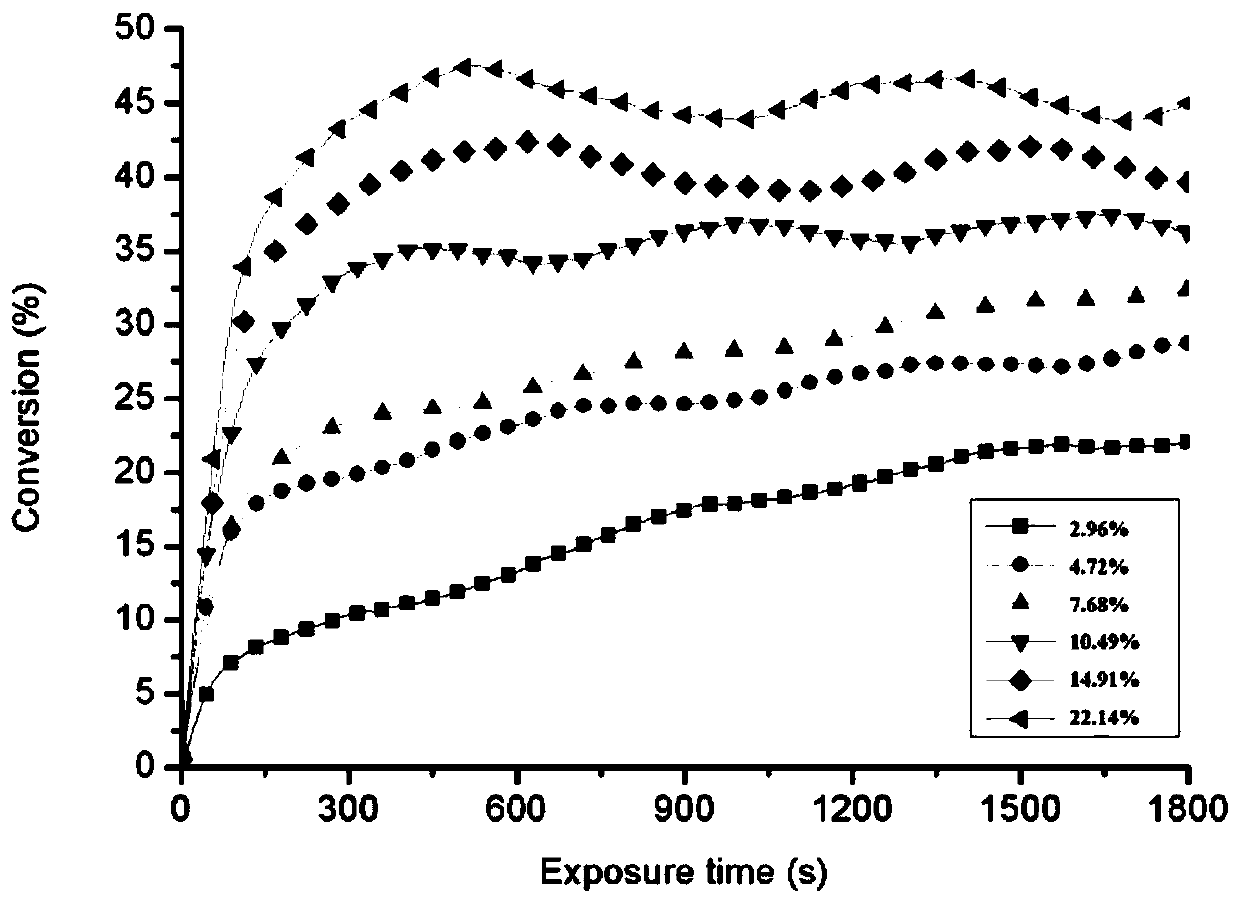
[0051] Prepare the molar ratio of photoinitiator 127 and allyl digose ether respectively as 2.96%, 4.72%, 7.68%, 10.49%, 14.91% and 22.14% of the mixture, evenly spread on the dried potassium bromide sheet, use Fu Kinetic curves of photopolymerization were obtained by Leaf transform real-time infrared (RT-IR). like image 3 As shown, the conversion rate of allyl disac...
Embodiment 3
[0053] Add 5 g of kojibiose (equivalent to 1 part of kojibiose) into a three-neck flask filled with 25 ml of pure water, stir and disperse evenly. Then add 5.291g of methyl chloride propene (equivalent to 4 parts of methyl chloride propene), adjust the pH to 9, and after magnetically stirring for 15 minutes, add 0.050 g of tetrabutylammonium perchlorate (equivalent to 0.02 parts of tetrabutyl perchlorate ammonium chlorate) was added to the reaction solution. The mixed solution was heated to 75° C., and the reaction was continued to stir for 24 hours. After extraction, it was washed and dried, and purified by chromatographic column to obtain allyl digose ether.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com