Polyformaldehyde all-solid-state polymer electrolyte prepared by in-situ ring-opening polymerization and application thereof
An all-solid-state polymer and ring-opening polymerization technology, applied in solid electrolytes, electrolytes, non-aqueous electrolytes, etc., can solve problems such as difficult practical application of lithium batteries, difficulty in compatibility with high-potential positive electrode materials, and low room temperature ionic conductivity. Improved interface stability and long-term cycle performance, excellent room temperature ionic conductivity, and high room temperature ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a glove box filled with argon, 4g of paraldehyde and 1.5g of succinonitrile were stirred for 5 minutes on a hot stage at 60°C and mixed evenly, then 0.375g of LiDFOB was added and stirred for 10 minutes to mix evenly, and the above mixed solution was taken 0.15 mL was added dropwise with cellulose as the support material; then LiCoO 2 As the positive electrode and lithium as the negative electrode, the porous support material soaked in the mixture above is placed between the positive and negative electrodes, and the assembled battery is heated at 60°C for 2 hours, and the mixture is polymerized in situ on the porous support material inside the battery to obtain an all-solid state polymer electrolyte (see figure 1 ), and then obtain an all-solid-state polymer lithium battery.
[0038] Depend on figure 1 It can be seen that the formed all-solid electrolyte is evenly attached to the cellulose non-woven separator.
Embodiment 2
[0040] Stir 2.5g 1,3,5-trioxane (TXE) and 1.5g succinonitrile on a hot stage at 80°C in a glove box filled with argon for 5 minutes and mix well, then add 0.375g g LiDFOB and continue stirring for 5 minutes Minutes to mix evenly, take 0.15mL of the above mixed solution and add it dropwise with cellulose as the support material; then use LiFeO 4 As the positive electrode and lithium as the negative electrode, the porous support material soaked in the mixture above is placed between the positive and negative electrodes, and the assembled battery is heated at 80°C for 5 hours, and the mixture is polymerized in situ on the porous support material inside the battery to obtain an all-solid state polymer electrolyte.
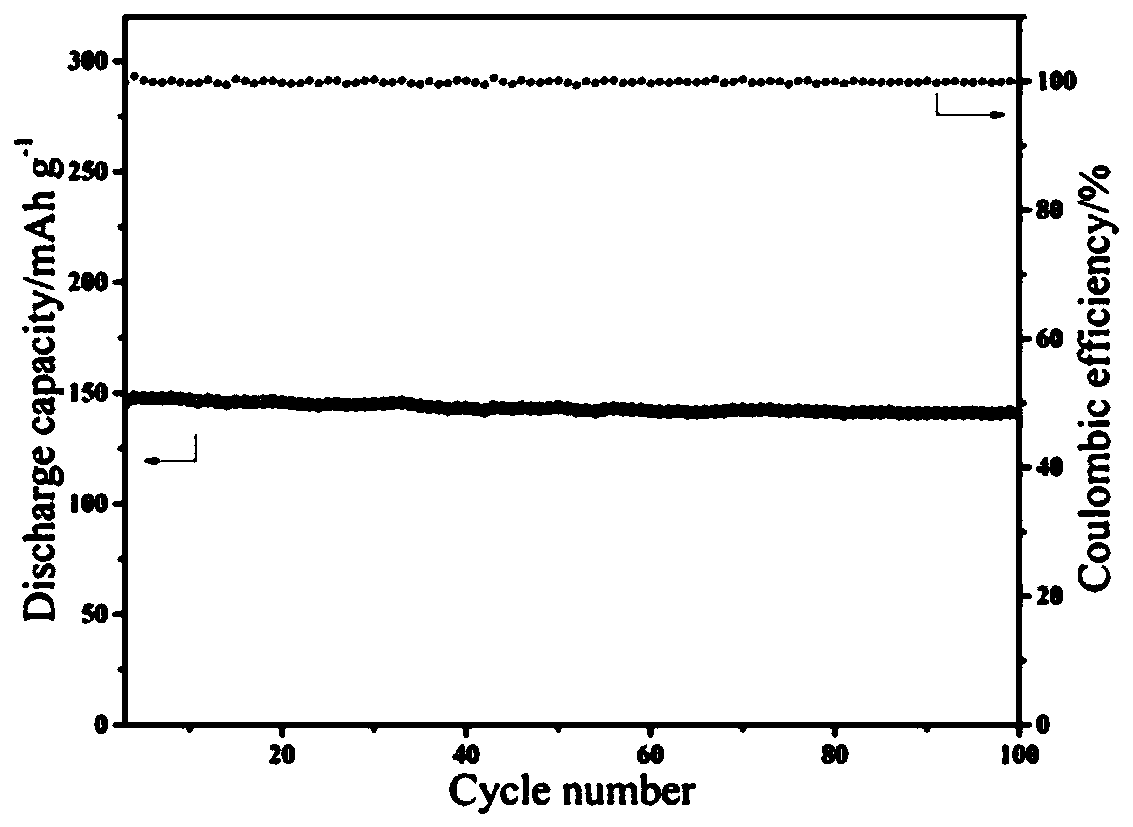
[0041] Use the above-mentioned all-solid-state polymer lithium battery to carry out the battery cycle test, the battery charge and discharge range is 2.75V-4V, the charge and discharge rate is 0.3C, and the test temperature is room temperature (see image 3 ). .
[...
Embodiment 3
[0044] Stir 2.5g 1,3,5-trioxane (TXE) and 2.5g succinonitrile on a hot stage at 80°C in a glove box filled with argon for 10 minutes, then add 0.375g LiDFOB and continue stirring for 5 Mix evenly within 1 minute, add 0.15 mL of the above mixed solution dropwise to cellulose as a support material; then use the ternary material as the positive electrode and lithium as the negative electrode, place the porous support material soaked in the mixture between the positive and negative electrodes, and assemble The completed battery is heated at 80°C for 5 hours, and the mixture is polymerized in situ on the porous support material inside the battery to obtain an all-solid polymer electrolyte.
[0045] The above-mentioned all-solid-state polymer lithium battery is used for battery cycle test, the battery charge and discharge range is 2.75V-4.3V, the charge and discharge rate is 0.2C, and the test temperature is room temperature (see Figure 4 ).
[0046] Depend on Figure 4 It can be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com