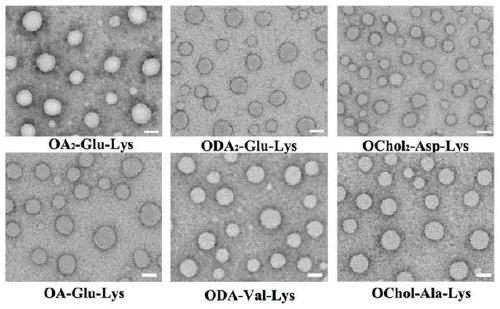
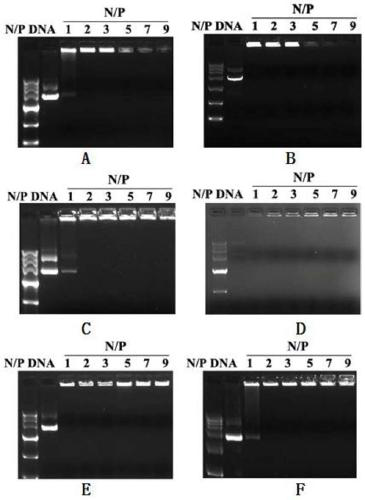
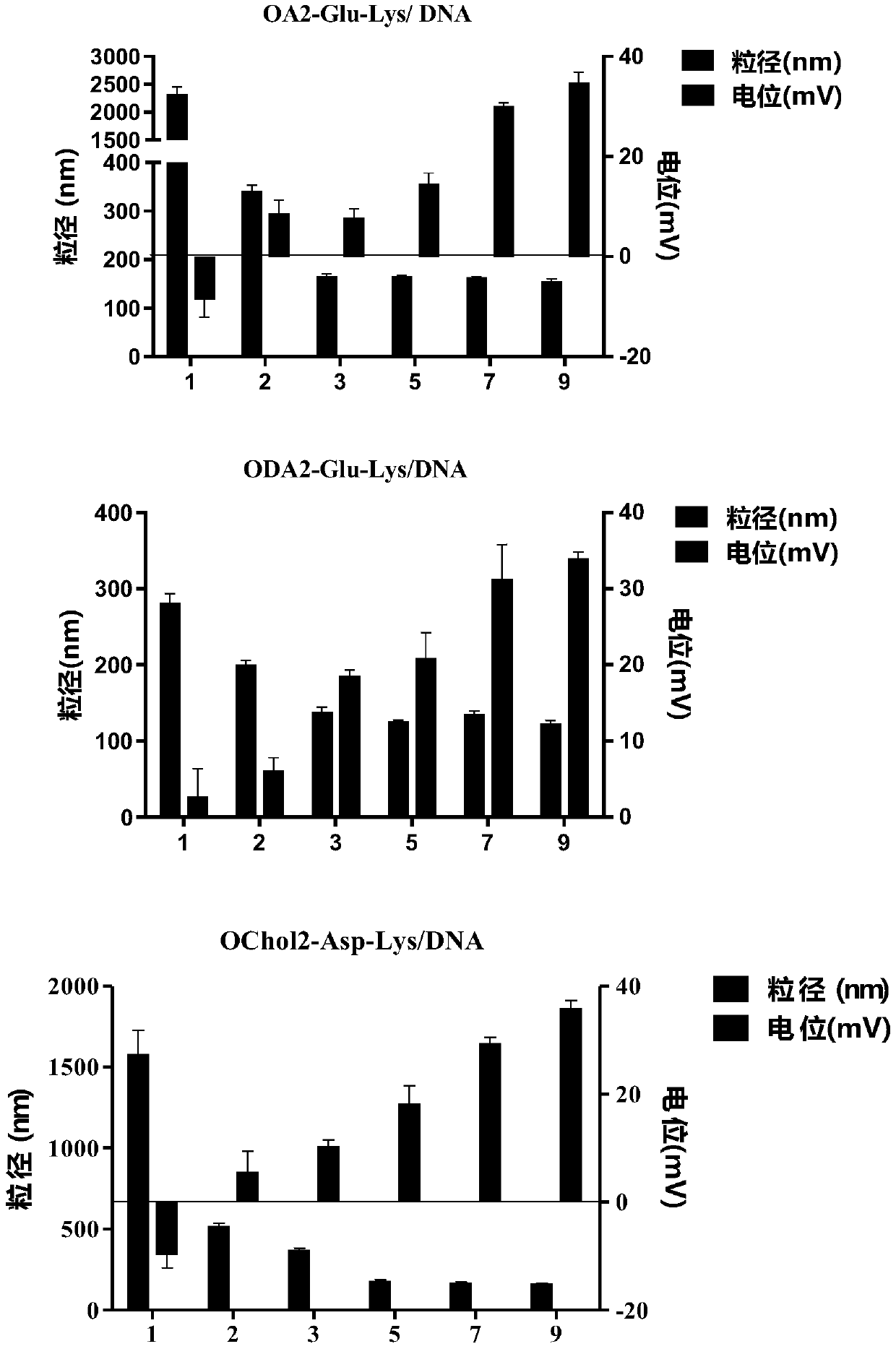
Unsaturated cationic lipid derivative, preparation method, and application in plasmid delivery system
A technology of cationic lipids and cationic liposomes, applied in liposome delivery, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of difficult DNA delivery and release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of Dioleyl Glutamate (OA 2 -Glu), the chemical structure is as follows:
[0076]
[0077] Dissolve L-glutamic acid (5.00g, 33.9mmol) in 200mL of anhydrous toluene, add p-toluenesulfonic acid (6.44g, 37.4mmol) with stirring, raise the temperature to 140°C, and reflux for 2h. After cooling to room temperature, oleyl alcohol (19.2 g, 71.4 mmol) was added, the temperature was raised to 150° C., and the mixture was refluxed overnight. After the reaction, the toluene was removed by rotary evaporation to obtain a dark brown oil. Dissolved in 300mL chloroform, washed with saturated aqueous sodium bicarbonate (200mL×2), washed with saturated brine (200mL×1), dried over anhydrous sodium sulfate, concentrated after suction filtration to obtain a dark brown oil, which was purified by column chromatography ( Petroleum ether: ethyl acetate = 10: 1) to obtain 6.40 g of a colorless transparent oil, yield: 54%.
[0078] 1 H NMR (300MHz, CDCl 3 ): δ(ppm)5.43–5.28(m,4H...
Embodiment 2
[0080] Preparation of Boc group-protected lysine glutamic acid dioleyl ester (OA 2 -Glu-Lys(Boc) 2 ), the chemical structure is as follows:
[0081]
[0082] Boc-Lys(Boc)-OH (484mg, 2.085mmol) was dissolved in 30mL of chloroform and placed at 0°C, EDCI (639mg, 3.335mmol) and HOBT (451mg, 3.335mmol) were added sequentially with stirring. After the addition was complete, transfer to room temperature and stir for 3 h to obtain reaction solution A; 2 -Glu (1.35 g, 2.085 mmol) was dissolved in 20 mL of chloroform, triethylamine (872 μL, 6.254 mmol) was added with stirring, and stirred at room temperature for 1 h to obtain reaction solution B. The reaction solution B was slowly dropped into the reaction solution A, and stirred overnight at room temperature. After the reaction, wash twice with an appropriate amount of water, twice with an appropriate amount of 10% citric acid aqueous solution, once with an appropriate amount of saturated saline, dry over anhydrous sodium sulfat...
Embodiment 3
[0085] Preparation of Dioleyl Lysine Glutamate (OA 2 -Glu-Lys), the chemical structural formula is as follows:
[0086]
[0087] will OA 2 -Glu-Lys(Boc) 2 (481mg, 0.493mmol) was placed at 0°C, and 30ml of hydrogen chloride-1,4-dioxane solution (4.0M concentration) was slowly added dropwise. After the reaction, the reaction solution was concentrated to obtain 335 mg of a yellow gel-like solid, yield: 80.2%.
[0088] 1 H NMR (500MHz, CDCl 3 ):δ(ppm)8.21(brs,2H,NH 2 ),7.87(brs,2H,NH 2 ),5.40–5.30(m,4H,CH 2 CHCHCH 2 ),4.55–4.39(m,1H,NH 2 CH),4.14–3.99(m,4H,COOCH 2 ),3.27–3.08(m,1H,NH 2 CH),2.68–2.40(m,2H,NH 2 CH 2 ),2.19–2.10(m,2H,CH 2 CO), 2.08–1.93 (m, 8H, CH 2 CHCHCH 2,2H,NHCHCH 2 ),1.76–1.55(m,4H,COOCH 2 CH 2 ,2H,NHCH 2 CH 2 CH 2 CH 2 ), 1.36–1.25 (m, 44H, CH 2(oleyl) ,2H,NH 2 CH 2 CH 2 CH 2 ,2H,NH 2 CH 2 CH 2 ), 0.88(t, J=7.1Hz, 6H, CH 2 CH 3 ). 13 C NMR (125MHz, CDCl 3 ): δ (ppm) 173.32 (1C, CH 2 COOCH 2 ), 171.44 (1C, NHCHCO), 169.47 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com