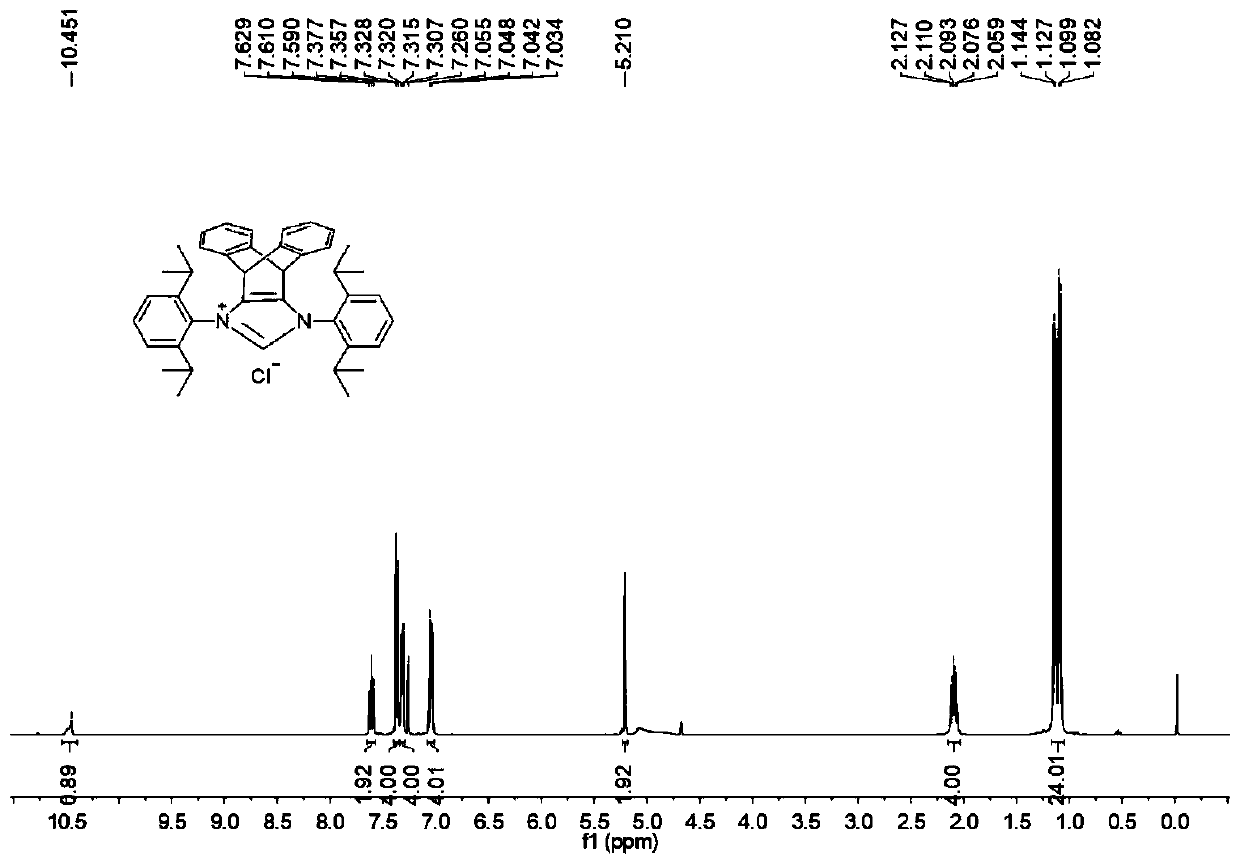
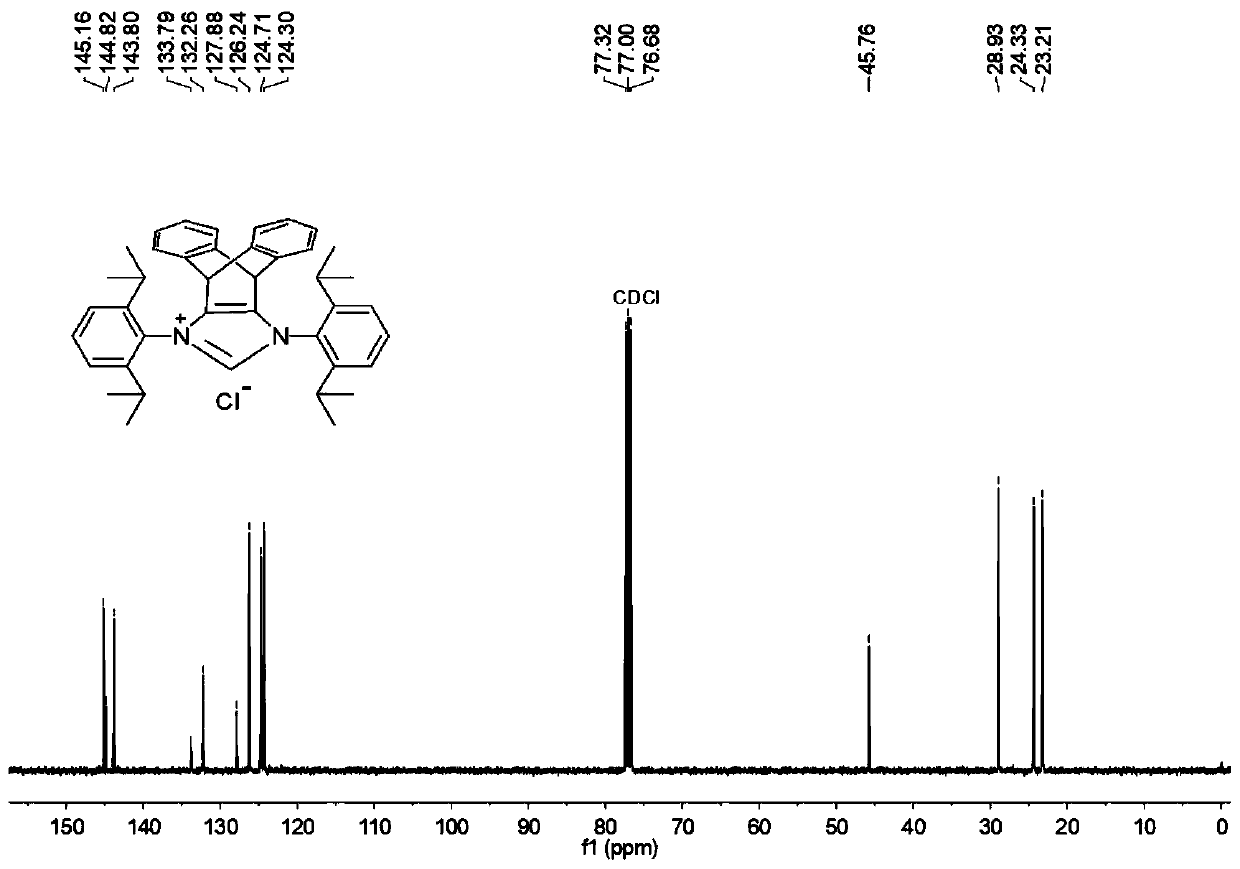
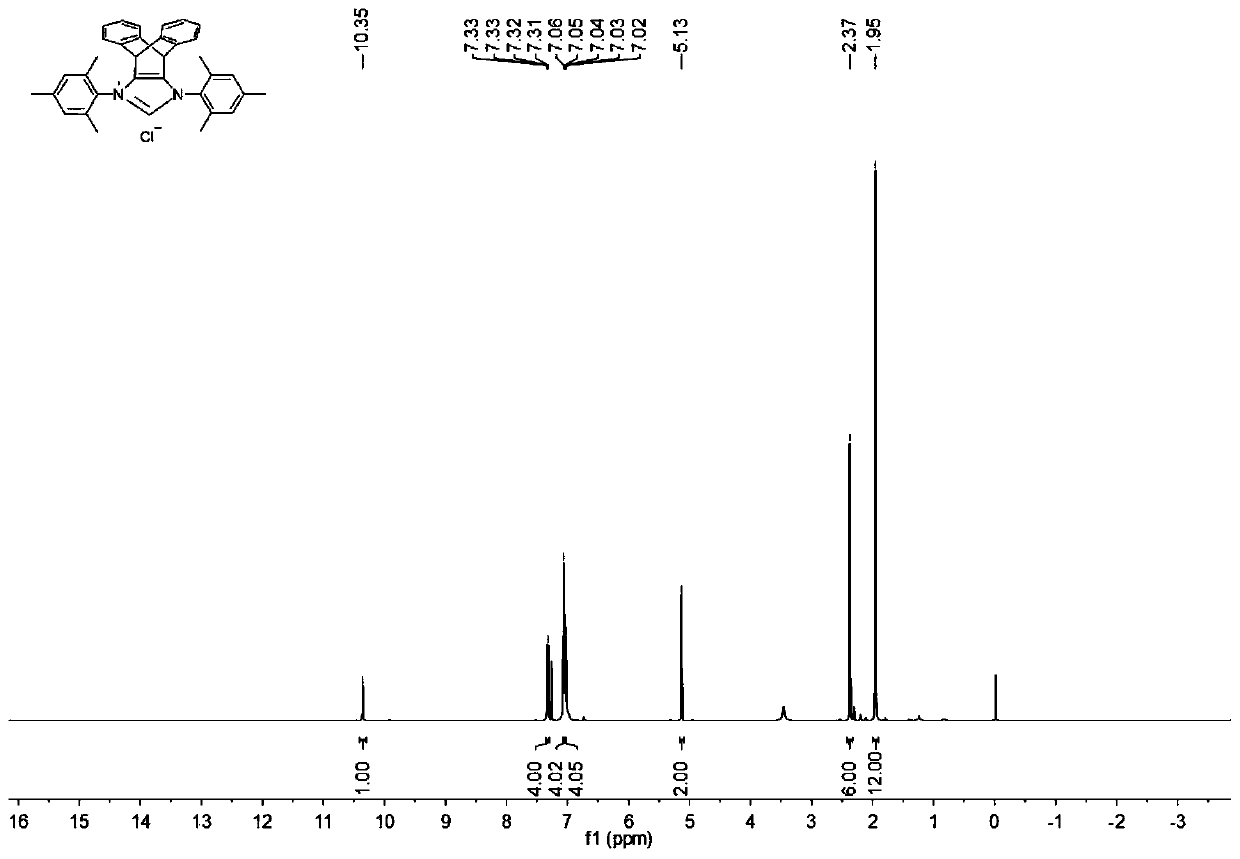
N-heterocyclic carbene palladium complex with ptycene structure and application thereof
A technology of complexes and pterenes, which is applied in the direction of palladium organic compounds, platinum group organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of no reactivity and achieve broad commercialization prospects and high Reaction yield, enhance the effect of industrialization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The chemical synthesis route of N-heterocyclic carbene palladium complex with pterene structure is shown below.
[0035] (1) Synthesis of Pterenone Compound A
[0036]
[0037] Under the protection of nitrogen, anthracene (1.78 g, 10.0 mmol) and vinylene carbonate (8.60 g, 100.0 mmol) were successively added into a 100 mL thick-walled bottle, and the mixture was refluxed at 180° C. for 8 h. After the reaction, the reaction solution was cooled to room temperature, methanol was added to the reaction solution and stirred. After a large amount of solid precipitated out of the reaction system, it was suction filtered. The solid was repeatedly washed with methanol and dried in vacuo to obtain white compound A with a yield of 82%.
[0038] (2) Synthesis of Pterene Diols Compound B
[0039]
[0040] Compound A (2.11g, 8.0mmol), 1,4-dioxane (90mL) and 4N NaOH solution (5ml) were added to a 250ml check flask, and the reaction was refluxed at 100°C for 2h. Cool to room t...
Embodiment 2
[0088] Example 2 N-heterocyclic carbene palladium complexes with pterene structure catalyzed Suzuki-Miyaura coupling reaction
[0089] The experimental steps for testing the catalytic activity of the N-heterocyclic carbene palladium complex with a pterene structure for the Suzuki-Miyaura coupling reaction are as follows:
[0090] Set up a control group experiment, 2-thiophene boronic acid (1.0mmol), 2-chloropyridine (1.0mmol) as substrate, mixed with sodium carbonate (2mmol), and tetrahydrofuran and water (1:3v / v, 4ml) as solvent Added into parallel reaction tubes, C1-C3, D1, E1 (0.1 mol% of the substrate) were respectively used as catalysts and added into parallel tubes. Then react in air at 80°C for 4h. After the reaction, the parallel reaction tubes were cooled to room temperature, and extracted by adding ethyl acetate and water for 2-3 times. The organic layer was dried with anhydrous sodium sulfate, the remaining reaction solution was evaporated by rotary evaporation, t...
Embodiment 3
[0100] Due to the high reactivity of thiophene boronic acid, the results in the comparison experiment can not fully reflect the high activity of the pterene skeleton structure catalyst, so a series of nitrogen-containing heterocyclic chlorides (1.0mmol) with lower activity, nitrogen-containing heterocyclic Cycloboronic acid (1.0mmol) was used as a substrate, mixed with sodium carbonate (2mmol), and added to parallel reaction tubes with tetrahydrofuran and water (1:3v / v, 4ml) as a solvent, and the N-heterocycle disclosed by the present invention Carbene palladium complexes C1-C3 (0.5 mol% of substrate) as catalysts were added to parallel tubes. Then react in air at 80°C for 0.5h. After the reaction, the parallel reaction tubes were cooled to room temperature, and extracted by adding ethyl acetate and water for 2-3 times. The organic layer was dried with anhydrous sodium sulfate, and the remaining reaction solution was evaporated by rotary evaporation. The separated product was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com