Cyclohexene-based hexanediamine preparation process
A preparation process, cyclohexene technology, applied in the direction of reductive alkylation preparation, ozonolysis preparation, including molecular sieve catalysts, etc., can solve the problems of dependence on imports, high toxicity of adiponitrile, high price, etc., to achieve clean routes and improve Catalyst stability and selectivity improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
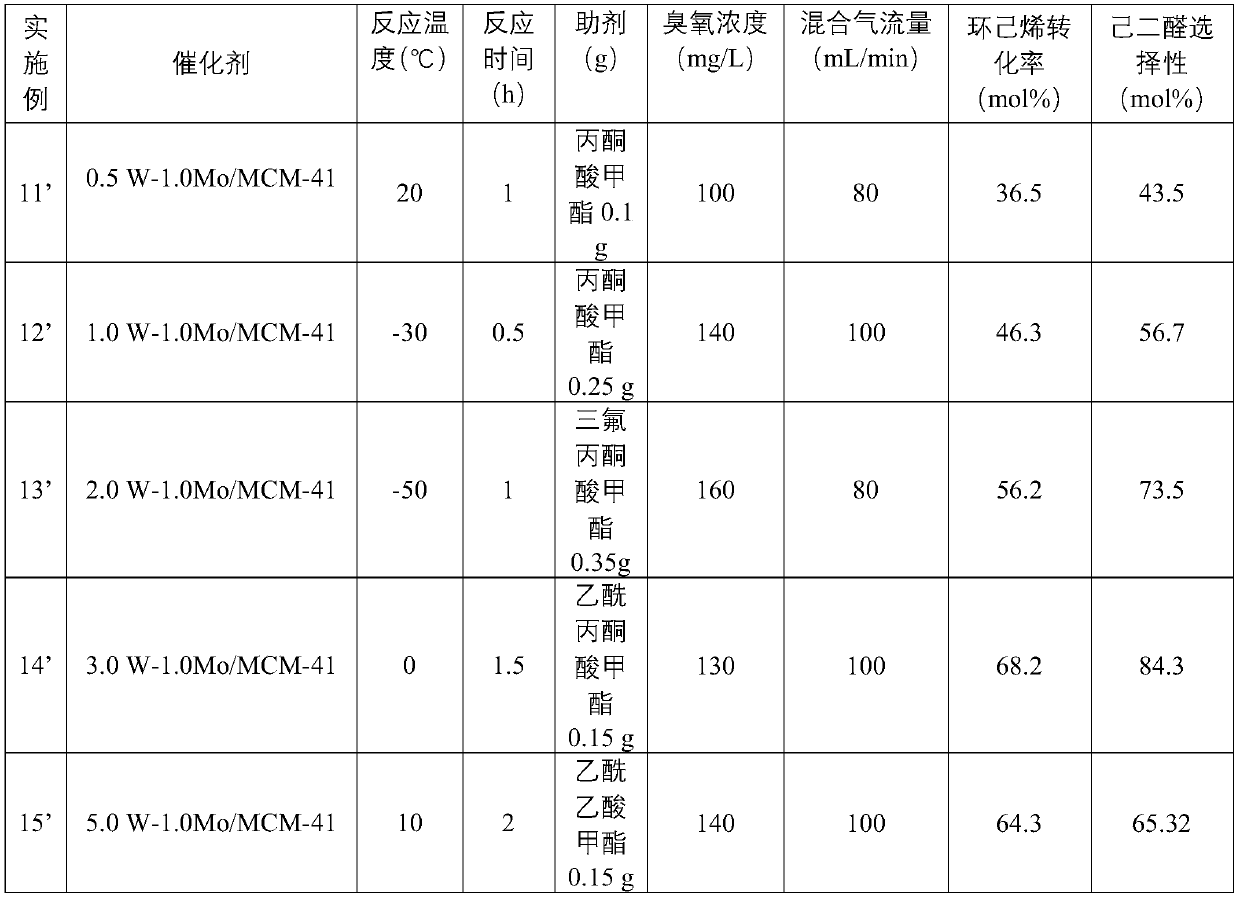
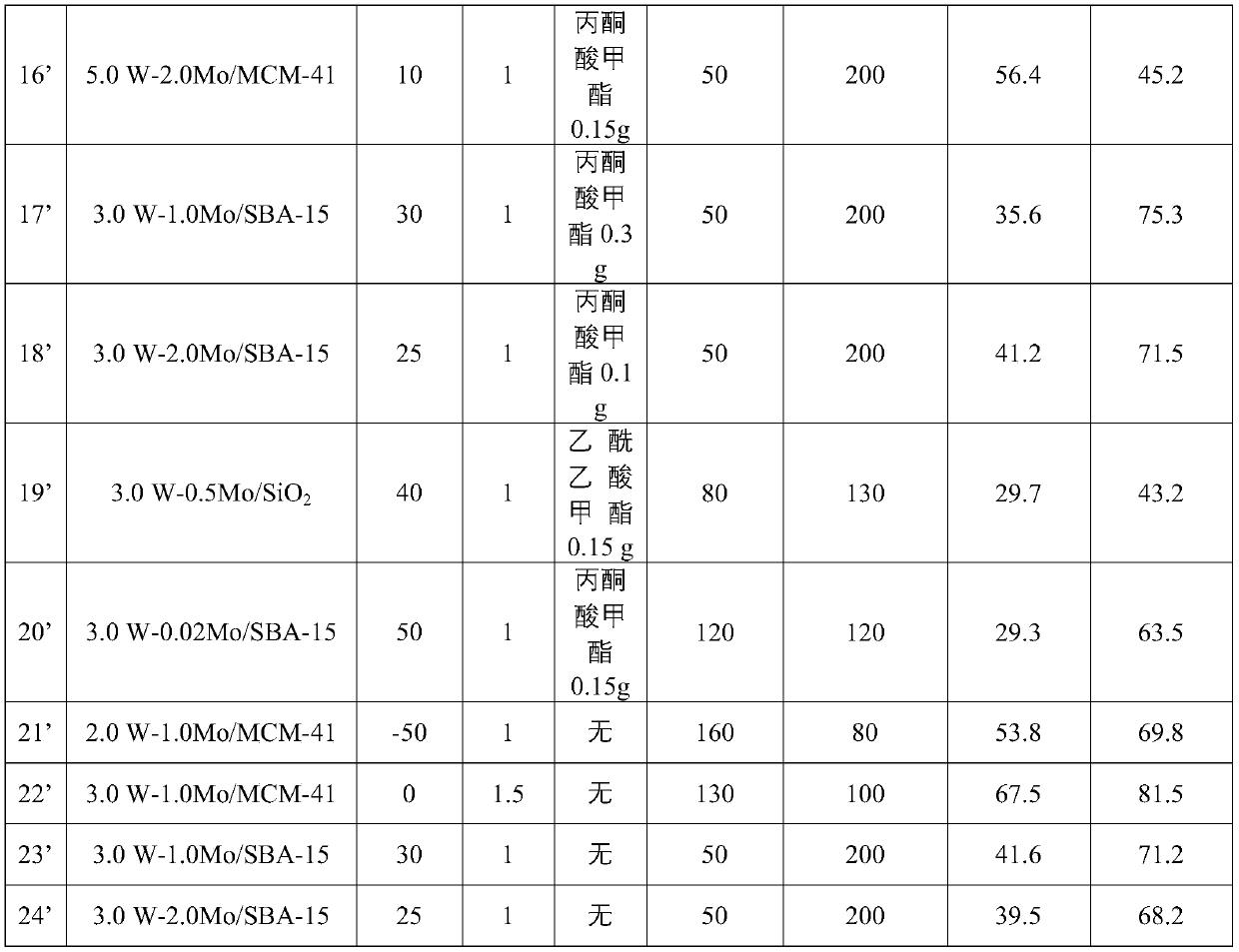
[0059] The preparation parameter of table 1 catalyst
[0060] Example catalyst Ammonium tungstate quality (g) Ammonium molybdate mass (g) Carrier mass (g) 1’ 0.5W-1.0Mo / MCM-41 0.07 0.18 10.00 2’ 1.0W-1.0Mo / MCM-41 0.14 0.18 10.00 3’ 2.0W-1.0Mo / MCM-41 0.28 0.18 10.00 4’ 3.0W-1.0Mo / MCM-41 0.42 0.18 10.00 5’ 5.0W-1.0Mo / MCM-41 0.69 0.18 10.00 6’ 5.0W-2.0Mo / MCM-41 0.69 0.37 10.00 7’ 3.0W-1.0Mo / SBA-15 0.42 0.18 10.00 8’ 3.0W-2.0Mo / SBA-15 0.42 0.37 10.00 9’ 3.0W-0.5Mo / SiO 2
[0061] Wherein, in the name of the catalyst aW-bMo / X, a represents the mass loading of the metal W element, b represents the mass loading of the metal Mo element, and X represents the support.
Embodiment 11
[0062] Embodiment 11' cyclohexene oxidation produces adipaldehyde
[0063] In a 250mL round-bottomed flask, add 1.0g of cyclohexene, 0.10g of methyl pyruvate, 25mL of acetonitrile, 0.5W-1.0Mo / MCM-41 catalyst (0.10g), rise to 20°C, and feed ozone with a concentration of The mixed gas of 100mg / L, described mixed gas is made up of ozone and oxygen, and described mixed gas flow rate is 80mL / min, is down to room temperature rapidly after reacting 1h, analyzes product composition with gas chromatograph.
[0064] Embodiment 12'-20' cyclohexene oxidation produces adipaldehyde
[0065] Carry out cyclohexene oxidation reaction according to the same step of embodiment 11', the specific differences and results of reaction conditions are shown in Table 2.
[0066] Example 21'
[0067] The preparation method is basically the same as that of Example 13', except that methyl trifluoropyruvate is not added, and the test results are shown in Table 2.
[0068] Example 22'
[0069] The prepara...
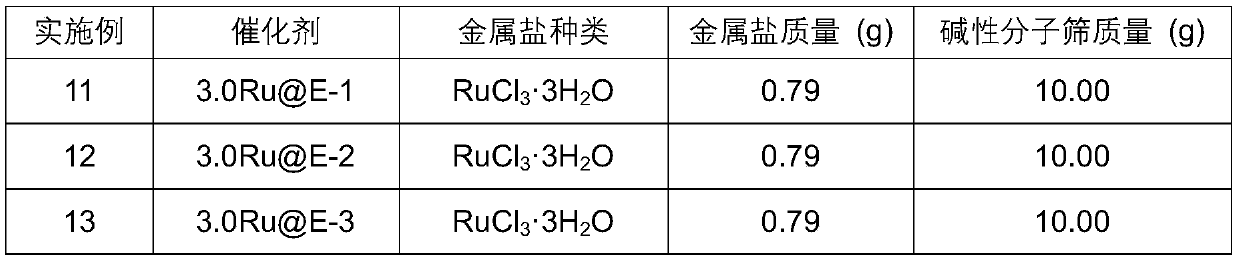
Embodiment 1-10
[0078] The preparation of embodiment 1-10 basic molecular sieve
[0079] Step 1): dissolving an alkali metal salt in water to obtain a precursor solution with a concentration of 0.2-0.6 mol / L, the alkali metal salt being selected from one of potassium nitrate, rubidium nitrate, and cesium nitrate;
[0080] Step 2): Weighing 30g molecular sieves, the molecular sieves are selected from one of NaX, NaY, KL, and NaBeta molecular sieves;
[0081] Step 3) According to the solid-to-liquid ratio of 10:1, use the alkali metal ion precursor solution in step 1) to carry out ion exchange on the molecular sieve weighed in step 2), exchange at 80°C for 4 hours, suction filter and wash 1. After drying, the obtained solid is roasted at 550° C. in a muffle furnace for 6 hours;
[0082] Step 4) Change the object of ion exchange in step 3) from the molecular sieve weighed in step 2) to the molecular sieve obtained after roasting, repeat step 3) twice to obtain a basic molecular sieve sample, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com