Aggregation-induced luminescent aniline derivative luminescent compound and preparation method thereof
An aggregation-induced luminescence, aniline derivative technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, chemical instruments and methods, etc., can solve the problems of health effects, time-consuming and labor-intensive, etc. Easy to obtain, linear detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Aniline Derivatives Luminescent Compounds with AIE Properties
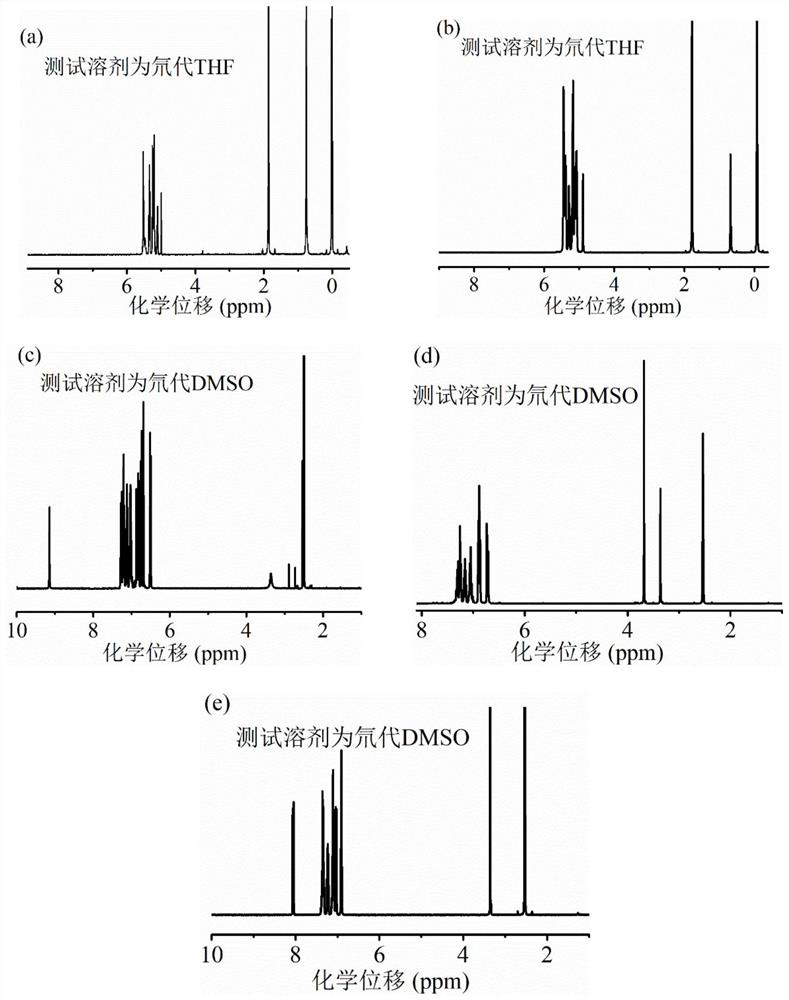
[0045] In this example, aniline derivative-based luminescent compounds containing different side groups were synthesized by a simple and effective method with a yield of 55-75%, and their chemical structures were fully characterized by H NMR and mass spectrometry.
[0046] The detailed synthesis method is as follows:
[0047] The first step: diphenylamine (diphenylamine: 0.642g, 3.8mmol; 4-bromo-diphenylamine: 0.939g, 3.8mmol; 4-hydroxyl-diphenylamine: 0.703g, 3.8mmol; 4-diphenylamine: 0.703g, 3.8mmol; Methoxy-diphenylamine: 0.757g, 3.8mmol and 4-nitro-diphenylamine: 0.813g, 3.8mmol), diphenylacetaldehyde (0.745g, 3.8mmol), Molecular sieves (1.011g), toluene (8ml) and tetrahydrofuran (2ml) were vigorously mixed and stirred for 20 minutes, and (±)-camphor-10-sulfonic acid (catalyst amount) was added; then the temperature was raised to 110°C, carry out stoxenamine reaction, keep 110°C and ...
Embodiment 2
[0051] Study on Photophysical Properties of Aniline Derivatives Luminescent Compounds Containing Different Side Groups
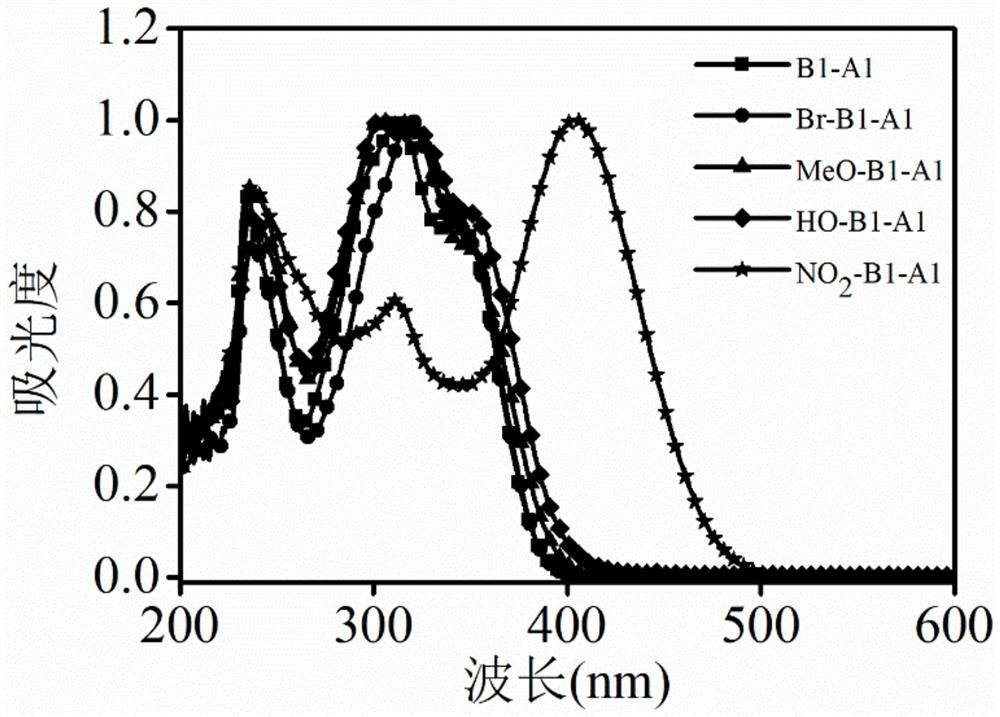
[0052] Such as figure 2 As indicated, the UV spectra of aniline derivatives modified with different side groups were tested. It is calculated that the aniline derivatives with different side groups have strong light absorption ability. Compounds B1-A1, Br-B1-A1, HO-B1-A1, MeO-B1-A1 and NO 2 -B1-A1 molar extinction coefficients are 20550, 23626, 27154, 24173 and 17900cm respectively -1 .mol -1 .L.
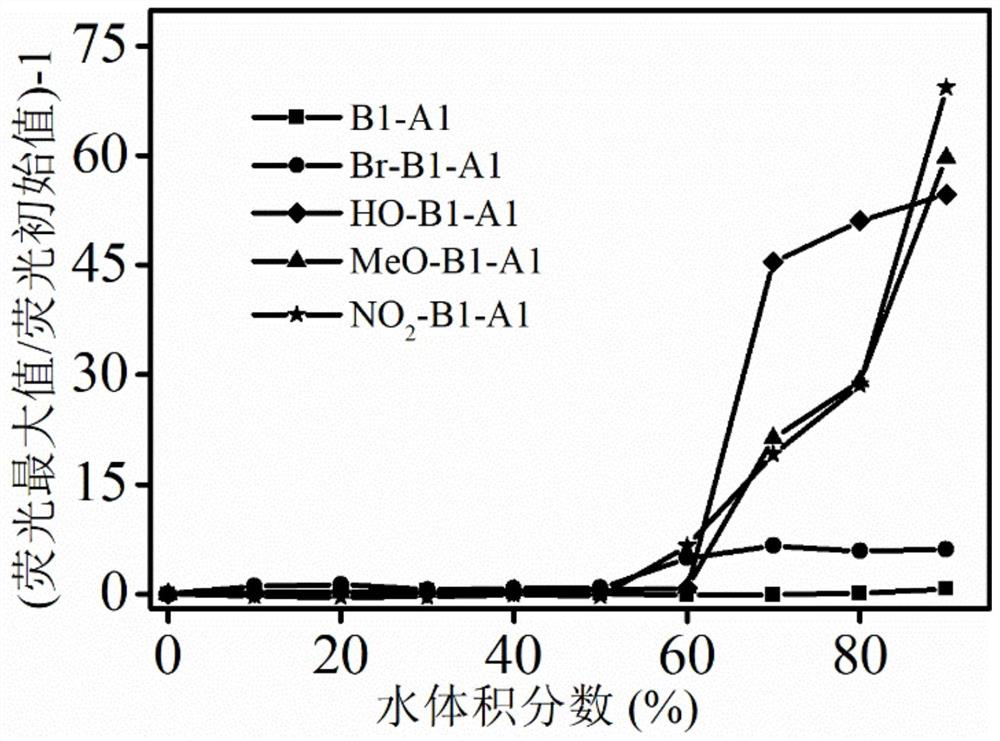
[0053] Further, the fluorescence emission behavior of aniline derivatives containing different side groups in pure THF solvent and THF / water mixed solvent was investigated. The fluorescence intensity of compounds B1-A1 and Br-B1-A1 did not change significantly with the increase of water volume fraction in THF / water mixed solvent; while compounds HO-B1-A1, MeO-B1-A1 and NO 2 -B1-A1 As the volume fraction of water in the THF / water mixed solvent increases, t...
Embodiment 3
[0055] Force-responsive Luminescence Study of Compound MeO-B1-A1
[0056] Take the compound MeO-B1-A1 obtained in Example 1, that is, 0.4 g of yellow-green crystals, and dry it in a vacuum oven at 40° C. for 2 hours. After drying is complete, the yellow-green crystals are placed in a mortar and divided into two equal parts.
[0057] Grind the right half well with a pestle and wait for comparison. Such as Figure 4 As shown, under the sunlight, the shape of the ground MeO-B1-A1 changed from yellow crystal to light yellow powder; under the irradiation of 365nm ultraviolet light, the color of the emitted light changed from cyan to cyan.
[0058] Studies have shown that the compound MeO-B1-A1 will have a significant change in luminescent color under the action of external force grinding, which can be used in force-responsive smart materials, preferably in force-stimuli-responsive materials for external force sensors, anti-counterfeiting labels, and information storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com