Aflatoxin B1 nano antibody immune adsorption material as well as preparation method and application thereof
An immunoadsorbent material and aflatoxin technology, applied in the field of preparation of immunoaffinity materials, can solve the problems of product instability, poor specificity and high price, and achieve the effects of high specificity, easy expression and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Construction of pET-30a-G8-Halo vector
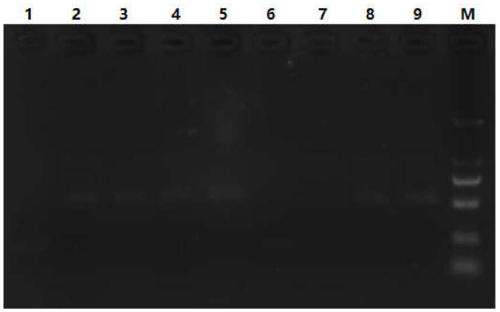
[0020] anti-AFB 1 Nanobody-encoding gene vector pET-25b-G8-DIG3 (the sequence disclosed in "One-step ELISA detection of aflatoxin B1 based on nanobody-alkaline phosphatase fusion protein", self-made in the laboratory) as a template , using G8-F0 and G8-R0 in Table 1 as primers for PCR amplification to obtain PCR products. PCR reaction 50ul system: ddH 2 O37.5ul, template (pET-25-G8-DIG lng / ul) 1ul, dNTP (2.5mM) 4ul, 5×PS Buffer 5ul, G8-F0 1ul, b72℃30s, a total of 25 cycles, the last 72℃10min fully extended. The G8(F0 / R0) PCR product was separated by 1% agarose gel electrophoresis to 409bp, and was produced with TaKaRa Fragment Purification Kit.
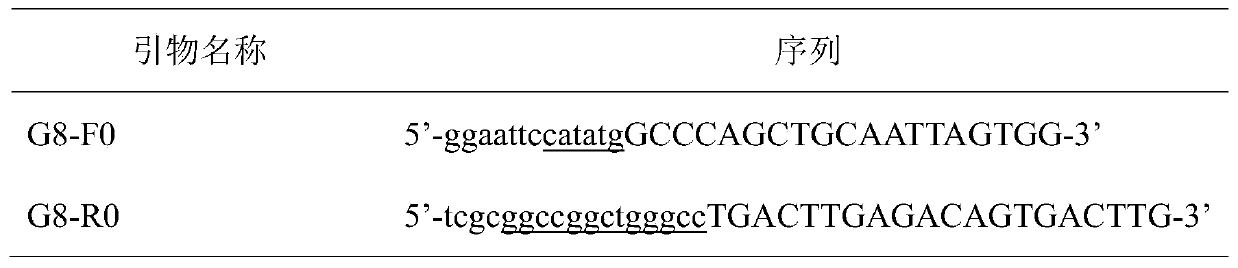
[0021] Table 1 PCR primers
[0022]
[0023]
[0024] Note: The underline is the enzyme recognition site
[0025] The PCR product encoding G8 and the plasmid vector pET-30a-T9-Halo ("One-step oriented immobilization of nanoparticles and its application for immunoglobulin ...
Embodiment 2
[0028] Example 2: Fusion Expression of G8-Halo Nanobodies
[0029] Transform the correctly sequenced pET-30a-G8-Halo vector into BL21(DE3) competent cells, pick a single colony from the plate and inoculate it in LB-K test tube medium, and culture it overnight at 220rpm at 37°C; Inoculate in 50mL LB-K medium according to 1% inoculation amount, shake culture at 37°C and 220rpm, when OD 600 When = 0.5, IPTG inducer was added to the culture to a final concentration of 0.5 mM, and then placed at 18° C. for induction culture for 12 h.
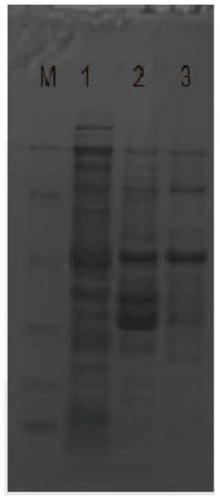
[0030] Centrifuge the culture after induction of expression in the previous step at 8000rpm for 20min, collect the cell pellet and resuspend the cell pellet with 1 / 10 volume of PBS buffer, and ultrasonically break the cell suspension for 10min until the broken cell suspension is clear, 4°C, 8000°C After centrifuging at rpm for 20min, the supernatant and the precipitate were collected respectively; SDS-PAGE was used to analyze the expression of the t...
Embodiment 3
[0031] Example 3: Immobilization and coupling of Halo-Tag protein
[0032]Take 50 μL of Halolink Resin (purchased from Promega, gel volume, the same below) into a 1.5 mL centrifuge tube, centrifuge at 3000 rpm for 1 min and discard the supernatant. Add 400 μL of binding buffer (100 mM Tris, 150 mM NaCl, 5% glycerol, pH 7.6) to the centrifuge tube in the previous step and mix thoroughly, centrifuge at 3000 rpm for 2 min, discard the supernatant, and repeat the operation 2 times. Resuspend Halolink Resin in 100 μL binding buffer, add 50 μL Halo-Tag-labeled protein and mix well, shake at room temperature for 2 hours to keep Halolink Resin suspended in the binding buffer. After centrifugation at 3000rpm for 2min, the supernatant was taken, and the protein concentration in the supernatant was measured by the Bradford method to calculate the coupling amount. Add 1mL wash buffer (100mM Tris, 150mM NaCl, 5% glycerol, 1mg / mL BSA pH 7.6) and mix thoroughly, shake at room temperature fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com