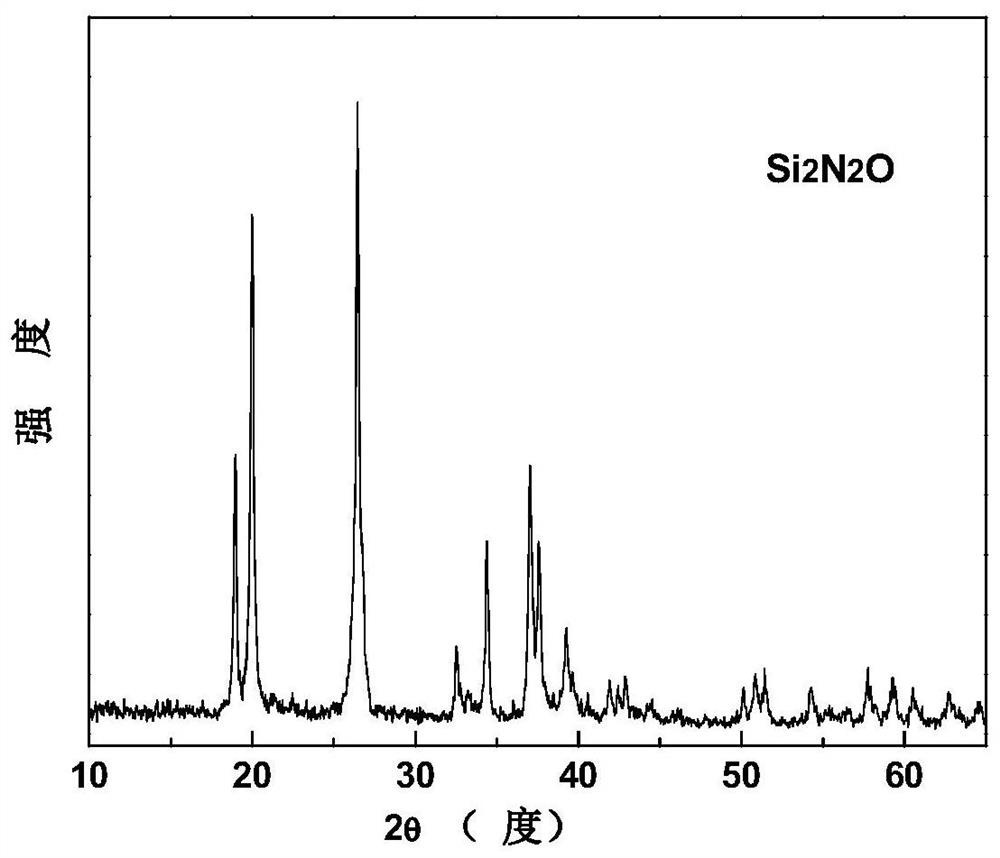
A kind of method for preparing silicon oxynitride powder under air atmosphere
A technology of silicon oxynitride and air atmosphere, which is applied in the field of preparation of inorganic non-metallic powder materials, can solve the problem of small phase gradient distance of pure silicon oxynitride, low yield of silicon oxynitride powder, and increased amount of silicon powder in the upper layer, etc. Problems, to achieve the effect of improving product purity, increasing ratio, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take silicon powder (I) with an average particle size of 1.12 μm and an oxygen content of 1.51% and loosely pack it into a ceramic saggar with a loose thickness of 60 mm; ) on the accumulation body, covering a thickness of 5mm. Place the above-mentioned ceramic sagger in the uniform temperature zone of the high-temperature furnace, raise the temperature to 1000°C at a heating rate of 10°C / min, and then raise the temperature to 1380°C at a heating rate of 5°C / min, and keep it warm for 3.5 hours to carry out the nitrogen oxidation synthesis reaction; after that Cool to room temperature with the oven. After the nitric oxide synthesis reaction, the lower product is white and fluffy, and the powder used for covering the upper layer is in a micro-sintered state. After removing the micro-sintered product of the upper layer and a small amount of product at the contact point between the edge and the ceramic sagger, the silicon oxynitride powder is obtained finished product.
...
Embodiment 2
[0025] Take silicon powder (I) with an average particle size of 1.93 μm and an oxygen content of 0.91% and loosely pack it into a ceramic saggar with a loose thickness of 40 mm; ) on the accumulation body, covering a thickness of 20mm. Place the above-mentioned ceramic sagger in the uniform temperature zone of the high-temperature furnace, raise the temperature to 1000°C at a heating rate of 20°C / min, and then raise the temperature to 1420°C at a heating rate of 4°C / min, and keep it warm for 4 hours to carry out the nitrogen oxidation synthesis reaction; after that Cool to room temperature with the oven. After the nitric oxide synthesis reaction, the lower product is white and fluffy, and the powder used for covering the upper layer is in a micro-sintered state. After removing the micro-sintered product of the upper layer and a small amount of product at the contact point between the edge and the ceramic sagger, the silicon oxynitride powder is obtained finished product.
[...
Embodiment 3
[0028] Take silicon powder (I) with an average particle size of 0.68 μm and an oxygen content of 1.96% and loosely pack it into a ceramic saggar with a loose thickness of 100 mm; ) on the accumulation body, covering a thickness of 10mm. Place the above-mentioned ceramic sagger in the uniform temperature zone of the high-temperature furnace, raise the temperature to 1000°C at a heating rate of 15°C / min, and then raise the temperature to 1400°C at a heating rate of 5°C / min, and keep it warm for 2.5 hours to carry out the nitrogen oxidation synthesis reaction; after that Cool to room temperature with the oven. After the nitric oxide synthesis reaction, the lower product is white and fluffy, and the powder used for covering the upper layer is in a micro-sintered state. After removing the micro-sintered product of the upper layer and a small amount of product at the contact point between the edge and the ceramic sagger, the silicon oxynitride powder is obtained finished product. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com