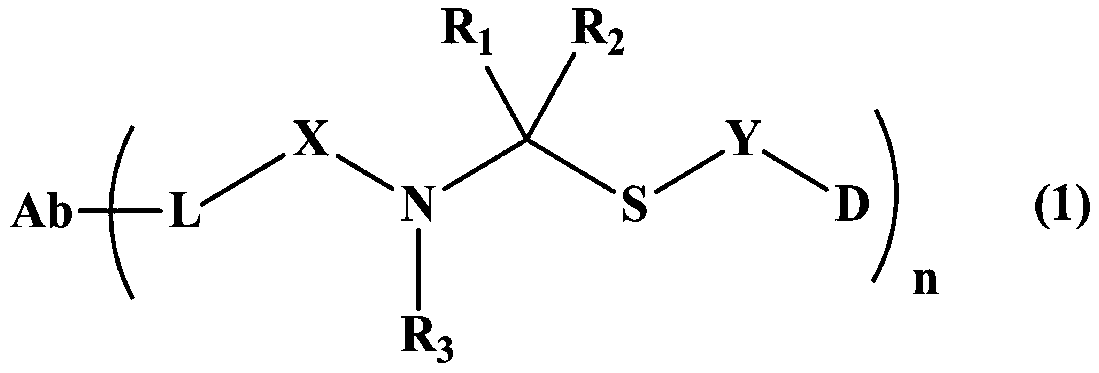
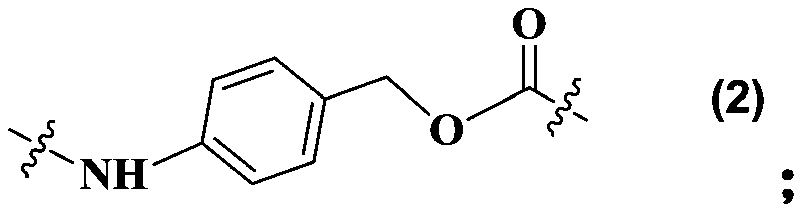
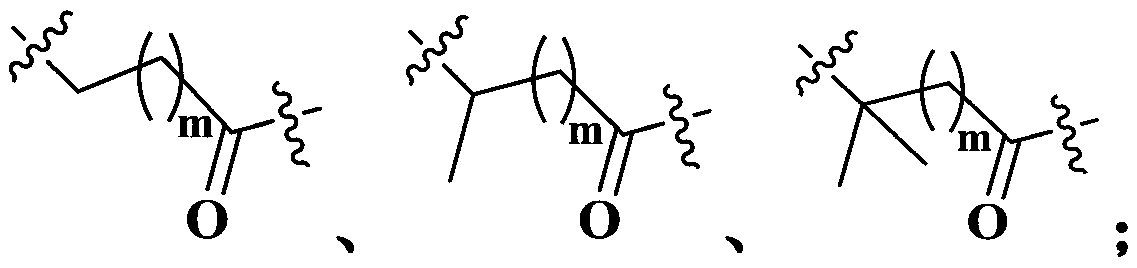
Maytansine antibody drug conjugate and application thereof
A technology of drug conjugates and antibody drugs, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of no bystander effect, inability to kill cancer cells, and drug efficacy effects, and improve the druggability. , enhance anti-tumor activity, enhance the effect of drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0052] General preparation example 1 : Preparation of VA-PAB
[0053]
[0054] Preparation of Cbz-VA(N-bianyloxycarbonyl-L-valyl-L-alanine) : Dissolve N-benyloxycarbonyl-L-valine (25.1g), TSTU (36.1g), DIPEA (38.7g) in acetonitrile (400mL) and stir at room temperature for 2.5h. The L-alanine solution was added to water (400 mL), added to the reaction system and stirred overnight at room temperature. Sampling was sent to LCMS, the reaction was good, concentrated hydrochloric acid was added dropwise to adjust the pH to 1. Spin to dry the solvent, add water (2000mL), and beat for 16h. After filtering and drying the filter cake, add ethyl acetate / dichloromethane / n-hexane = 500mL / 500mL / 1000mL to make slurry for 16h. Filtered, washed twice with n-hexane, and dried to obtain 30.7 g of the product, with a yield of 93%. LC-MS: (M+H)+322.5
[0055] Preparation of Cbz-VA-PAB (N-bianyloxycarbonyl-L-valyl-L-alaninamide-biancohol) : Dissolve Cbz-VA (30g), EEDQ (46g), PAB (p-ami...
preparation example 2
[0057] General preparation example 2 : Preparation of VA-PAB-NH-Ph-CH-DM1
[0058]
[0059] Preparation of Teoc-VA-PAB (trimethylsilylethoxycarbonyl-L-valyl-L-alaninamide-biancohol) : Teoc-OSU (2-(trimethylsilyl)ethoxycarbonyloxysuccinimide) (1.9g), VA-PAB (2.0g), DIPEA (2.7g) were dissolved in DMF (30mL) at room temperature Stir for 16h. Sampling was sent to LCMS, the reaction solution was spin-dried, and the solid was added to 400 mL of water and sonicated for 1 h. Filtered, washed twice with n-hexane, and dried to obtain 3.0 g of the product, with a yield of 62.5%. LC-MS: (M+H)+437.6
[0060] Teoc-VA-PAB-NH 2 Preparation of (trimethylsilylethoxycarbonyl-L-valyl-L-alaninamide-benamide) prepare : Teoc-VA-PAB (3.0g), NPC (6.4g), DIPEA (2.70g) were dissolved in DMF (50mL) and stirred at room temperature for 2h. Ammonium acetate (5.4g) dissolved in ethanol (50mL) was added to the reaction system and stirred at room temperature for 16h, the sample was sent to LCM...
preparation example 3
[0064] General preparation example 3 : Preparation of HS-Pr-VA-PAB-NH-Ph-CH-DM1
[0065]
[0066] Preparation of Bn-S-S-Pr-VA-PAB (bianthioether propionamide-L-valyl-L-alaninamide-biancohol) : Dissolve Bn-S-S-Pr (bianthioether propionic acid), TSTU (2.28g), DIPEA (3.68g) in DMF (200mL) and stir at room temperature for 5h. Add VA-PAB (2.52g) to the system and stir at room temperature for 16h, take a sample and send it to LCMS, spin the reaction solution to dryness, add DCM / ethyl acetate=400mL / 400mL to beat the solid for 16h. Filter and wash twice with n-hexane, and dry to obtain 2.70 g of the product with a yield of 62.5%. LC-MS: (M+H)+503.5
[0067] Bn-S-S-Pr-VA-PAB-NH 2 The preparation of (biansulfide propionamide-L-valyl-L-alaninamide-bianamide) prepare : Dissolve Bn-S-S-Pr-VA-PAB (2.7g), NPC (4.89g), DIPEA (2.0g) in DMF (80mL) and stir at room temperature for 2h. Ammonium acetate (4.0g) dissolved in ethanol (80mL) was added to the reaction system and stirred...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com