Molten salt production method for co-producing high-purity magnesium hydroxide, magnesium carbonate and nitrogen-potassium fertilizer
A magnesium hydroxide and production method technology, applied in the direction of magnesium hydroxide, magnesium carbonate, nitrogen fertilizer, etc., can solve the problems that the purity of potassium nitrate and the content of magnesium ions are difficult to meet the specified requirements, lack of scientific basis, unreasonable, etc., and achieve economic benefits Appreciable, high utilization rate of process raw materials, and the effect of reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
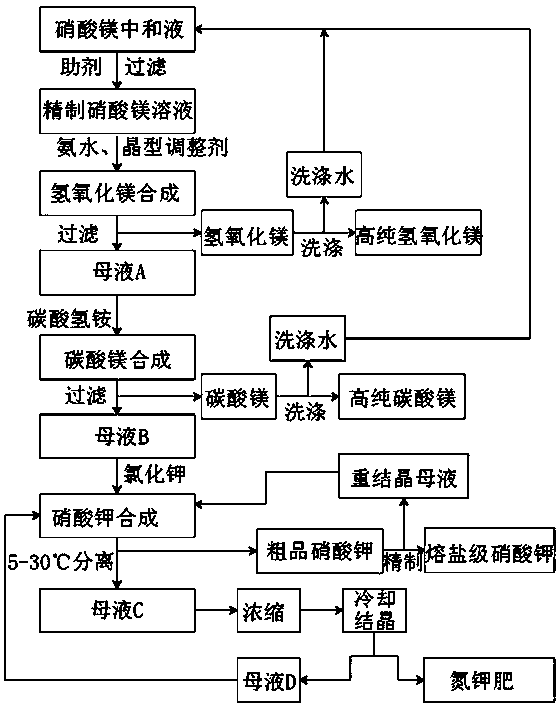
[0021] Example 1, step 1, react the magnesium slag after extracting lithium from the salt lake with dilute nitric acid with a concentration of 40-60%, and use magnesium hydroxide washing water or magnesium carbonate washing water to dilute and lower the temperature to control the reaction temperature below 83°C, and the obtained magnesium nitrate Neutralizing solution, after adding additives to remove heavy metals, filter to obtain refined magnesium nitrate solution 1573.6Kg, mass concentration 47%;
[0022] Step 2: Add 606Kg of 30% ammonia water (excess 7%) and refined magnesium nitrate solution into a high-speed stirring reaction pot, raise the temperature to 90°C, and then add 0.1% crystal form regulator to react. After the reaction is completed, filter to obtain granules Diameter 0.5-2 micron magnesium hydroxide crude product 275.6Kg and ammonium nitrate mother liquor A, after detecting the residual magnesium ion concentration in the solution, it can be known that the conve...
Embodiment 2
[0027] Example 2, step 1, react the magnesium slag after lithium extraction from the salt lake with dilute nitric acid with a concentration of 40-60%, and use magnesium hydroxide washing water or magnesium carbonate washing water to dilute and lower the temperature to control the reaction temperature below 83°C, and the obtained magnesium nitrate Neutralizing solution, after adding additives to remove heavy metals, filter to obtain refined magnesium nitrate solution 3287.2Kg, concentration is 45%;
[0028] Step 2, add the prepared 1247Kg (10% excess) 30% ammonia water and refined magnesium nitrate solution into the high-speed stirring reaction pot, raise the temperature to 90°C and add 2% crystal form modifier to react, and filter after the reaction is completed to obtain Magnesium hydroxide crude product 550.9Kg of particle diameter 0.5-2 microns and ammonium nitrate mother liquor A, after detecting residual magnesium ion concentration in the solution, it can be known that the...
Embodiment 3
[0033] Example 3, step 1, react bitter soil powder with dilute nitric acid with a concentration of 40-60%, and use magnesium hydroxide washing water or magnesium carbonate washing water to dilute and lower the temperature to control the reaction temperature below 83°C, and the obtained magnesium nitrate neutralization solution After adding additives to remove heavy metals, filter to obtain refined magnesium nitrate solution 3081.7Kg, concentration 48%;
[0034] Step 2, add the prepared 1430Kg (5% excess) 25% ammonia water and refined magnesium nitrate solution into the high-speed stirring reaction pot, raise the temperature to 90°C and add 3% crystal form regulator to react, and filter after the reaction is completed to obtain Magnesium hydroxide crude product 537.2Kg and ammonium nitrate mother liquor A of particle diameter 0.5-2 microns, after detecting residual magnesium ion concentration in the solution, it can be seen that the conversion rate of magnesium ion in this step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com