Catalyst for preparing multi-carbon product by electro-reduction of carbon dioxide and carbon monoxide and preparation method and application thereof
A carbon dioxide and carbon monoxide technology, applied in the field of electrocatalysis, can solve the problems of narrow reaction window and low activity, and achieve the effects of high selectivity, high reaction activity and simple and easy preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
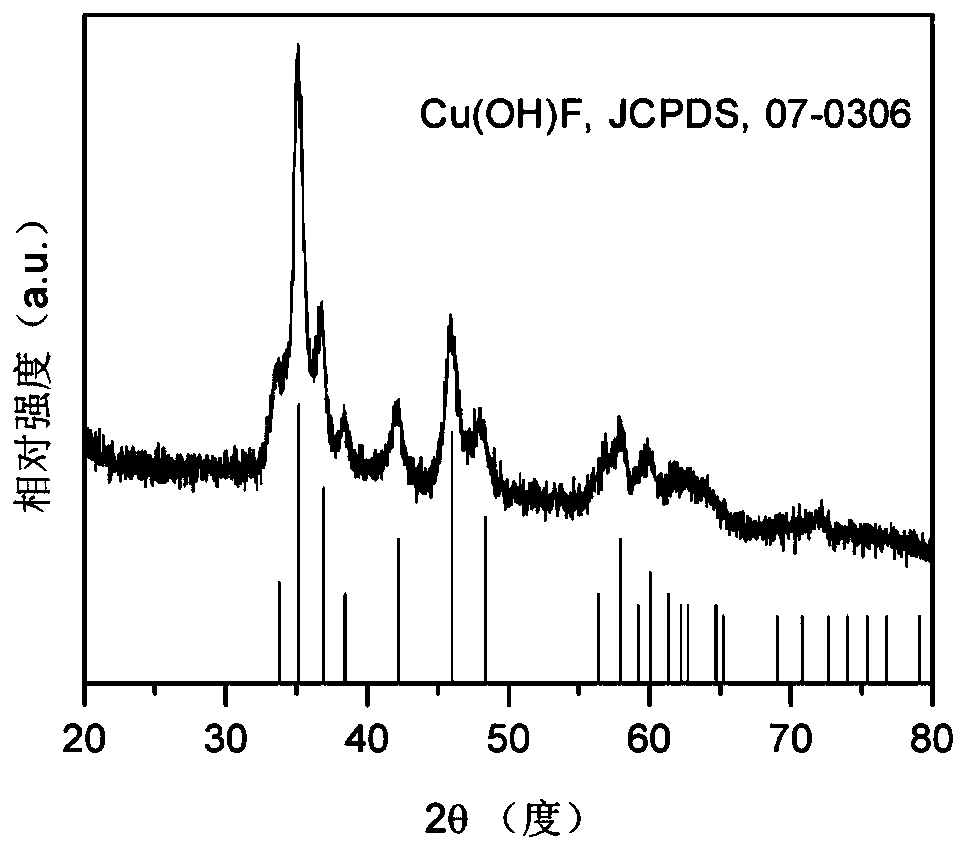
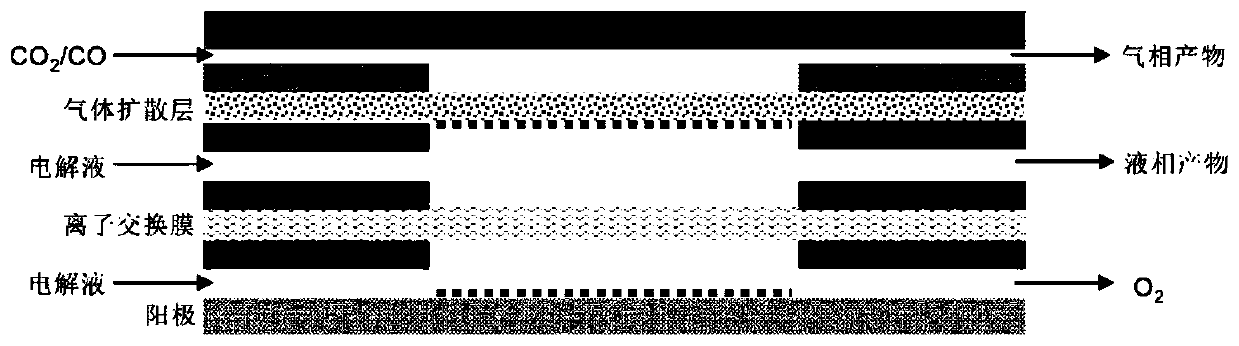
[0037] 0.2mmol NH 4 HF 2 and 0.2mmol Cu(NO 3 ) 2 ·3H 2O was dissolved in 50 mL of DMF, stirred vigorously for 15 min, then transferred to a 100 mL PTFE-lined stainless steel autoclave, sealed, and heat-treated at 160 °C for 4 h; cooled, washed with deionized water and ethanol, and dried to obtain Cu(OH )F precursor, the X-ray diffraction pattern of Cu(OH)F is as follows figure 1 shown; then Cu(OH)F was loaded on the gas diffusion layer with a load of 0.5 mg cm -2 ;Finally, the fluorine-modified copper electrocatalyst (F-Cu) was obtained by electroreduction at -0.6Vvs.RHE in 1.0M KOH electrolyte for 5min. The catalyst is used as the cathode, the nickel foam is used as the anode, and the saturated Ag / AgCl electrode is used as the reference electrode, and the reaction is carried out in a flow electrolytic cell. The configuration of the flow electrolytic cell is as follows: figure 2 Shown; cathode chamber and anode chamber are 1.0M KOH electrolyte; carbon dioxide in 50mL mi...
Embodiment 2
[0039] 1.0mmol NH 4 HF 2 and 0.5mmol Cu(OAc) 2 Dissolve in 100mL DMF, stir vigorously for 15min, then transfer to a 200mL PTFE-lined stainless steel autoclave, seal, heat at 180°C for 3h; cool, wash with deionized water and ethanol, and dry to obtain Cu(OH) F precursor; then Cu(OH)F was loaded on the gas diffusion layer with a loading of 0.25 mg cm -2 ;Finally, the F-Cu catalyst was obtained by electroreduction at -0.7V vs. RHE for 5min in 2.5M NaOH electrolyte. The catalyst was used as the cathode, the nickel foam was used as the anode, and the saturated Ag / AgCl electrode was used as the reference electrode, and the reaction was carried out in a flow electrolytic cell; both the cathode chamber and the anode chamber were 2.5M NaOH electrolyte; -1 The flow rate is passed into the cathode, and different negative currents are applied for 0.5h.
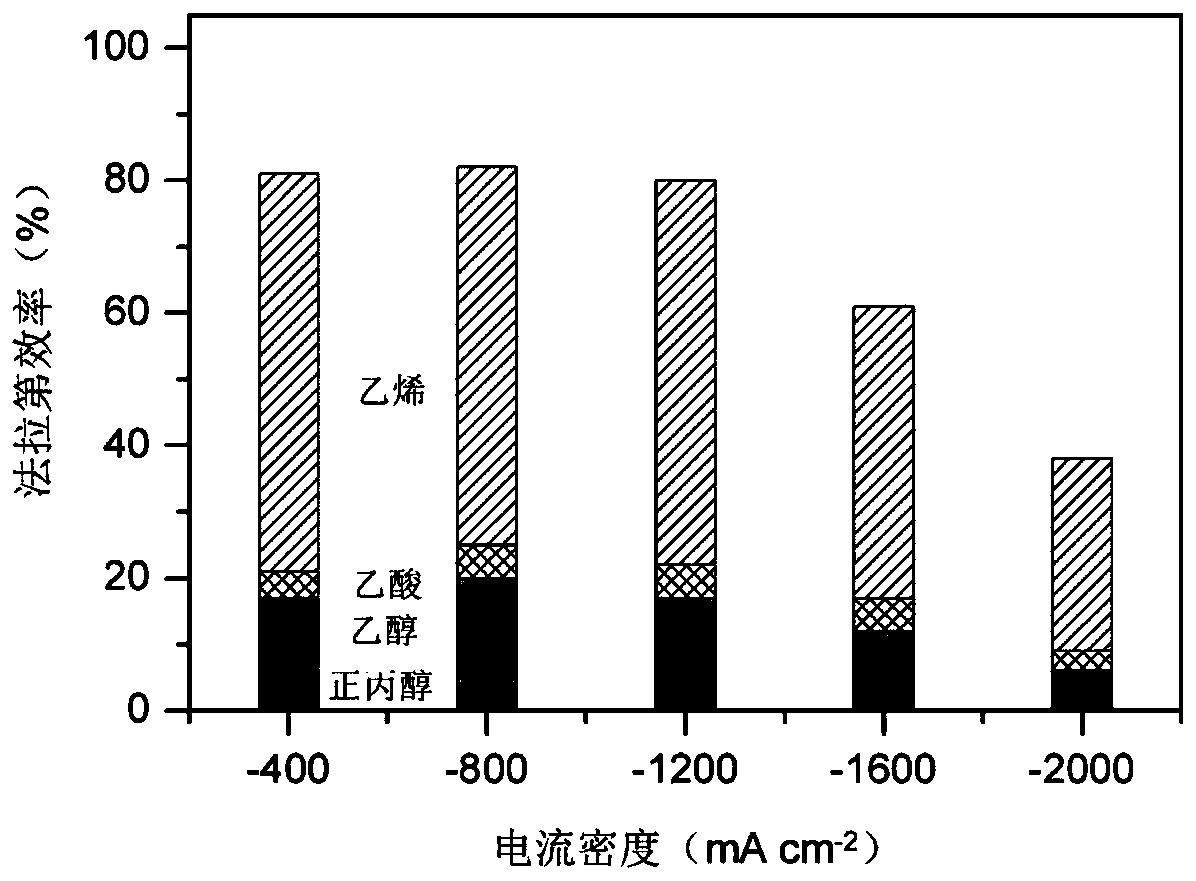
[0040] Such as image 3 As shown, change the current density applied to the F-Cu catalyst from -400 to -1200mA cm -2 , the faradai...
Embodiment 3
[0042] 0.3mmol NH 4 HF 2 and 0.2mmol CuNO 3 ·3H 2 O was dissolved in 50 mL of DMF, stirred vigorously for 15 min, then transferred to a 100 mL PTFE-lined stainless steel autoclave, sealed, and heat-treated at 160 °C for 6 h; cooled, washed with deionized water and ethanol, and dried to obtain Cu(OH )F precursor; then Cu(OH)F is supported on the gas diffusion layer with a loading of 1.0 mg cm -2 ;Finally, the F-Cu catalyst was obtained by electroreduction at -1.5V vs. RHE for 5min in 0.75M KOH electrolyte. The catalyst is used as the cathode, the nickel foam is used as the anode, and the saturated Ag / AgCl electrode is used as the reference electrode, and the reaction is carried out in a flow electrolytic cell; both the cathode chamber and the anode chamber are 0.75M KOH electrolyte; carbon dioxide is passed into the cathode at different flow rates , apply -1600mA cm -2 Current response 0.5h.
[0043] Such as Figure 4 As shown, when the carbon dioxide flow rate is 20mL m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com