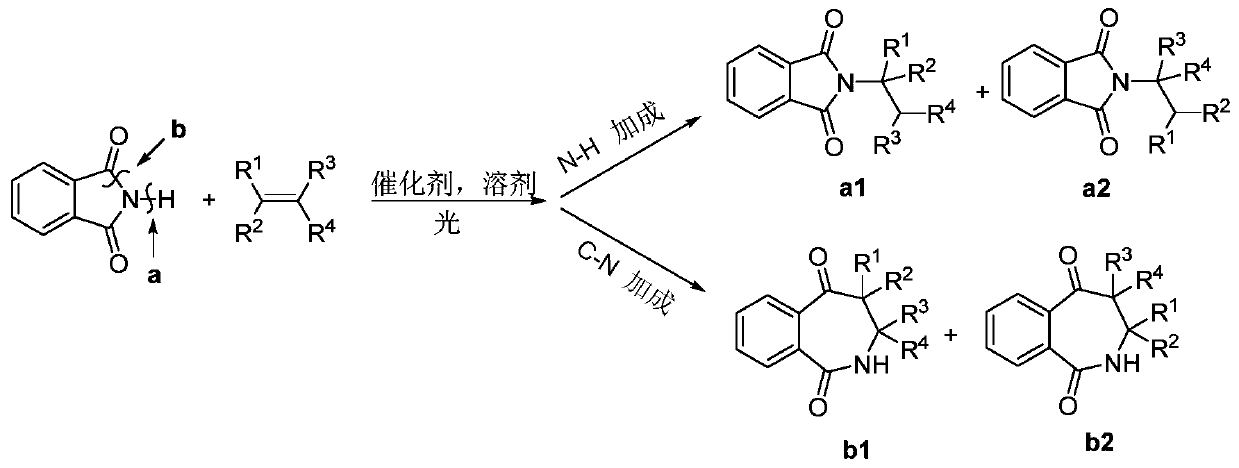
Method for generating C-N addition organic amine product by photocatalytic olefin addition amination reaction
An amination reaction and organic amine technology, which is applied in the field of photocatalytic olefin addition amination reaction to generate C-N addition organic amine products, can solve the problems of complex steps, low atom economy, low yield and the like, and achieves fast reaction rate , The effect of high product selectivity and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
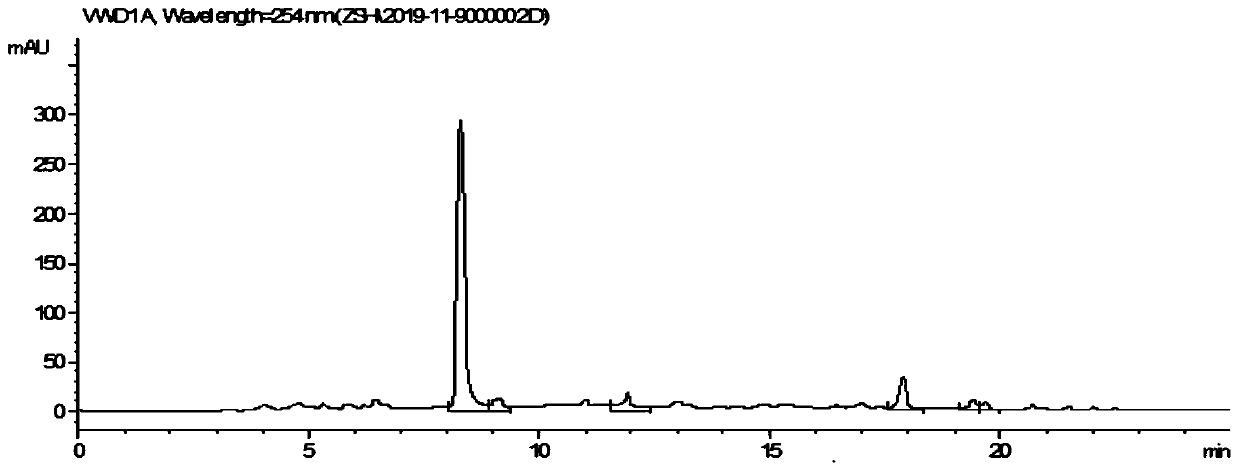
[0036] Dissolve phthalimide (199.7mg, 1.36mmol), cyclohexene (1.5mL, 14.6mmol), iron tetraphenylporphyrin (2mg), triethylamine (0.5mL) and water (3mL) in anhydrous acetonitrile (26 mL). The internally cooled photoreaction bottle was kept at 30°C, protected by nitrogen, and reacted for 2 hours under the conditions of a 250W high-pressure mercury lamp. After the reaction is over, the solvent and excess alkene are distilled off under reduced pressure, and the crude product is extracted with dichloromethane, concentrated and then used for column chromatography separation column chromatography (silica gel, 200-300 mesh; developing agent, petroleum ether, ethyl acetate ester) to obtain 272.4mg of 1,3,4,4a,5,11a-hexahydro-6H-dibenzo[b,e]azepine-6,11(2H)-dione, from the HPLC spectral analysis Product yield and selectivity all reach 99.0% (the HPLC before crossing column sees image 3 ), separated by a chromatographic column (of the product 1 H NMR as Figure 4 , 5), the separation...
Embodiment 2
[0040] Phthalimide (200.3mg, 1.36mmol), 4-methyl-1-cyclohexene (0.25mL, 2.04mmol), tetraphenylporphyrin iron (2mg), triethylamine (4.0mL ) and water (3 mL) were dissolved in anhydrous acetonitrile (25 mL). The internally cooled photoreaction bottle was kept at 30°C, protected by nitrogen, and reacted for 2 hours under the conditions of a 250W high-pressure mercury lamp. 1,3,4,4a,5,11a-hexahydro-6H-dibenzo[b,e]azepine-6,11(2H)-dione was obtained, the HPLC yield was 93.1%, and the selectivity was 93.1% (HPLC of crude product such as Figure 6 ).
[0041]
[0042] 2-Methyl-1,3,4,4a,5,11a-hexahydro-6H-dibenzo[b,e]azepine-6,11(2H)-dione
Embodiment 3
[0044] Phthalimide (203.0mg, 1.36mmol), tetramethylethylene (0.25mL, 2.04mmol), tetraphenylporphyrin iron (2mg), triethylamine (4.0mL) and water (3mL) Dissolve in anhydrous acetonitrile (25 mL). The internally cooled photoreaction bottle was kept at 30°C, protected by nitrogen, and reacted for 2 hours under the conditions of a 250W high-pressure mercury lamp. 3,3,4,4-Tetramethyl-3,4-dihydro-1H-benzo[c]azepine-1,5(2H)-dione was obtained with an HPLC yield of 94.3% and a selectivity of 94.3% % (HPLC of crude product such as Figure 7 ,).
[0045]
[0046] 3,3,4,4-Tetramethyl-3,4-dihydro-1H-benzo[c]azepine-1,5(2H)-dione
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap