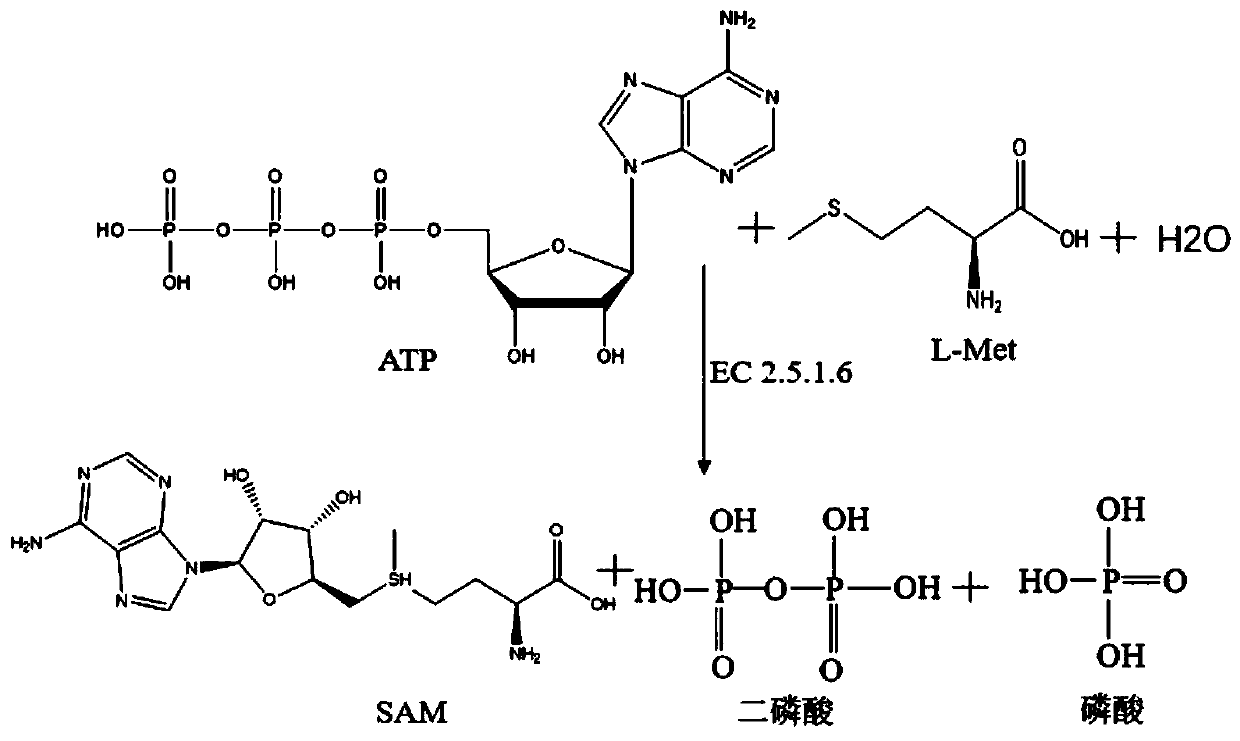
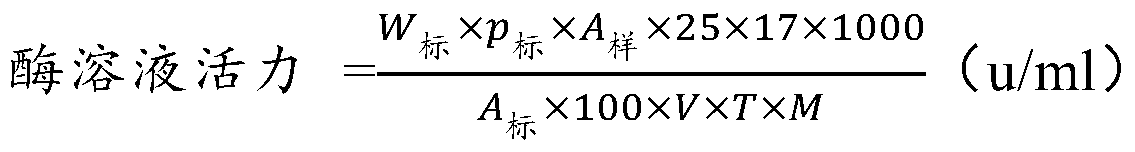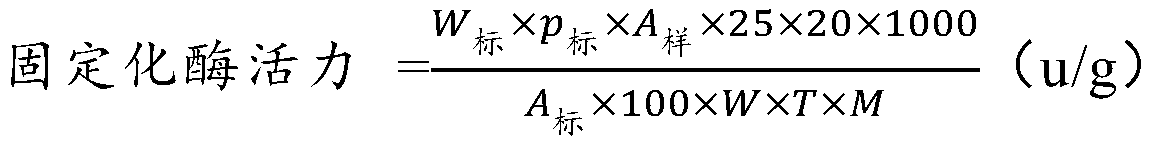S-adenosyl-L-methionine synzyme mutant and preparation method using same
A technology for adenosylmethionine and methionine, which is applied in the field of S-adenosylmethionine synthetase mutants and its preparation, can solve the problems of low product concentration, inability to scale up applications, and product Inhibition of large problems, to achieve the effect of low enzyme activity decline, high specific activity, and small product inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of prokaryotic expression strain of S-adenosylmethionine synthetase MATI derived from rat
[0052] Download the amino acid sequence of S-adenosylmethionine synthase MATI derived from rat liver in GenBank (SEQ ID NO.1 in this article, corresponding to GenBank accession number: NP_036992.2), and provide it to Beijing Qingke Biotechnology Co., Ltd. Total gene synthesis of encoding nucleic acid (using E. coli preferred codons). The C-terminal of the synthetic gene has a His tag, and it is constructed into the prokaryotic expression vector pET30a(+). The prokaryotic expression vector restriction site: Nde I at the 5' end, Xho I at the 3' end. Pass the constructed plasmid pET30a(+)-MATI through CaCl 2 Transformed into Escherichia coli expression strain BL21(DE3) by heat shock transformation method, spread on LB solid medium plate containing 50 μg / ml Kanamycin, and cultivate overnight at 37°C, the colony grown on the plate is S-adenosylmethylthio Amin...
Embodiment 2
[0054] Example 2: Purification and immobilization of rat-derived S-adenosylmethionine synthetase (MATI)
[0055] Using the His tag carried in the MATI recombinant protein, the supernatant obtained in Example 1 was treated with activated IDA resin (purchased from Anolon (Beijing) Biotechnology Co., Ltd., specific model: His.Bind Resin, Ni-charged). Carry out protein purification, the specific method is as follows: 4 ℃, 10000r / min, centrifuge the fermentation broth for 10min, discard the supernatant, collect the bacterial cells, the bacterial cells are repeatedly washed twice with phosphate buffer (pH 8.0, 0.1mol / L), centrifuged Afterwards, the cells were concentrated 5 times and resuspended in 20 ml of phosphate buffer (pH 8.0, 0.1 mol / L). The above-mentioned treated bacterial liquid was placed in ice water for ultrasonic crushing until clarification, the ultrasonic crushing conditions were: working for 2s, interval of 5s, ultrasonic power 500W. The crushed lysate was centrifu...
Embodiment 3
[0059] Example 3: Construction of MATI prokaryotic expression strain E.coli BL21(DE3) / pET30a(+)-MATI error-prone mutation library
[0060] Use the pET30a(+)-MATI recombinant plasmid as a PCR template, and conventional T7F / R as a universal primer (primer sequence: T7F: 5'-TAATACGACTCACTATAGGG-3'T7R: GCTAGTTATTGCTCAGCGG) for error-prone PCR amplification of the MATI gene, and adjust the PCR Mg in the amplification reaction system 2+ , Mn 2+ , dCTP and dTTP oligonucleotide concentrations, so that the base mismatch rate of the mutant library is only 2 / 1000, that is, it is guaranteed that only 1 to 2 amino acids are mutated in a mutant.
[0061] Error-prone PCR reaction system:
[0062]
[0063] Error-prone PCR reaction conditions: pre-denaturation at 95°C for 5 minutes; then denaturation at 94°C for 30 seconds, annealing at 56°C for 1 minute, and extension at 72°C for 1.5 minutes, a total of 25 cycles; finally, extension at 72°C for 10 minutes.
[0064] Sampling 2 μL of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com