PPK2 protein and application thereof as 30kd standard substance for polyacrylamide gel electrophoresis
A protein and gene-encoding technology, applied in the field of bioengineering, can solve problems such as unreported, and achieve the effects of non-degradable, long half-life and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Bioinformatics prediction
[0032] After obtaining the amino acid sequence of the PPK2 protein from the NCBI database (WP_AGG33389.1), the secondary structure of the PPK2 protein was predicted using the PSIPRED website (http: / / web.expasy.org / protparam); using the ExPASy website (http: / / bioinf. cs.ucl.ac.uk / psipred / ) to predict basic physicochemical properties of PPK2.
Embodiment 2
[0033] Example 2: Whole plasmid digestion and sequencing
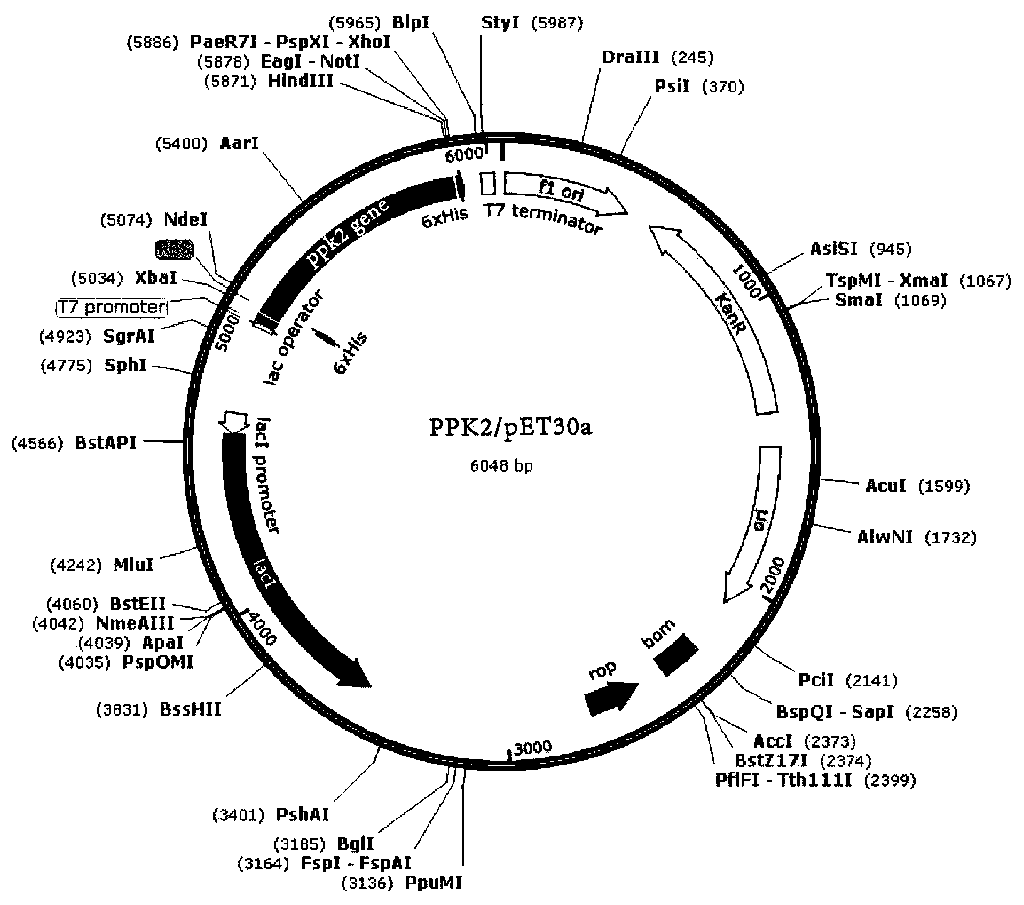
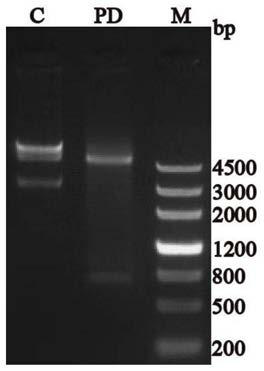
[0034] Recombinant expression plasmid PPK2 / pET30a synthesized by Beijing Aoke Dingsheng Biotechnology Co., Ltd. was digested with restriction endonucleases NdeΙ and HindⅢ to carry out double-enzyme digestion identification of the plasmid, and the results of DNA agarose gel electrophoresis experiments should have A 5250bp band and a 798bp band; at the same time, the PPK2 / pET30a recombinant expression plasmid was handed over to Jinweizhi Biotechnology Co., Ltd. to complete the sequencing of the target gene PPK2. Finally, confirm the accuracy of the recombinant expression vector.
Embodiment 3
[0035] Example 3: PPK2 protein test expression and identification
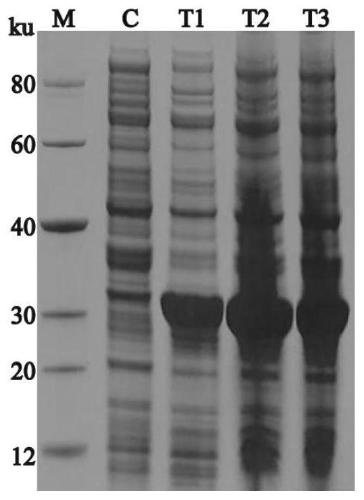
[0036] The recombinant expression plasmid PPK2 / pET30a constructed above was transformed into Escherichia coli BL21 (DE3) competent cells, and then spread evenly on LB plates (containing 50 μg·mL -1 kanamycin), and then placed in a 37°C incubator overnight. Pick a single clone from the transformed plate and inoculate it into 4ml of LB medium (containing 50μg·mL -1 Kanamycin), cultivated in a constant temperature shaker at 37°C, 220r / min, until OD 600 When the value is 0.5-0.8, add IPTG to the test tube culture solution to make the final concentration 0.2mM·L -1 , and then placed at 16°C, 25°C, and 37°C to induce expression. Centrifuge the induced culture medium at 12,000 rpm for 5 minutes, remove the supernatant, add PBS buffer to resuspend the pellet, and finally add SDS-PAGE loading buffer to heat the sample at 100°C for 10 minutes, then centrifuge to take the supernatant for SDS-PAGE Electrophoresis. Ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com