Nitrogen heterocyclic compound, display panel and display device
A nitrogen heterocyclic compound and display panel technology, applied in organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve the problems of poor coverage tightness, inability to meet high refractive index, inability to achieve close packing between molecules, etc. The effect of improving external quantum efficiency, improving luminous efficiency, and increasing polarizability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
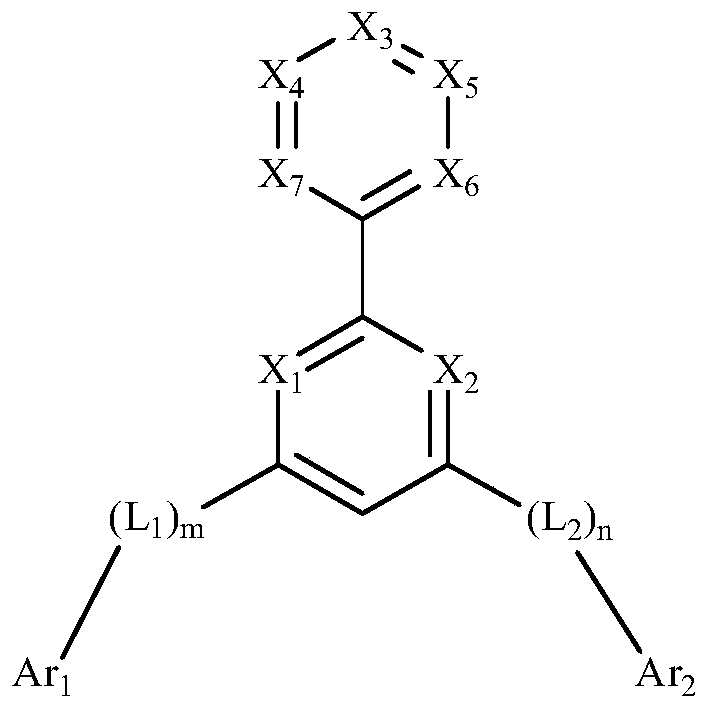
[0048] According to one embodiment of the nitrogen heterocyclic compound of the present invention, the nitrogen heterocyclic compound is selected from any one of the following compounds:
[0049]
[0050]
[0051]
[0052]
[0053]
[0054]
[0055]
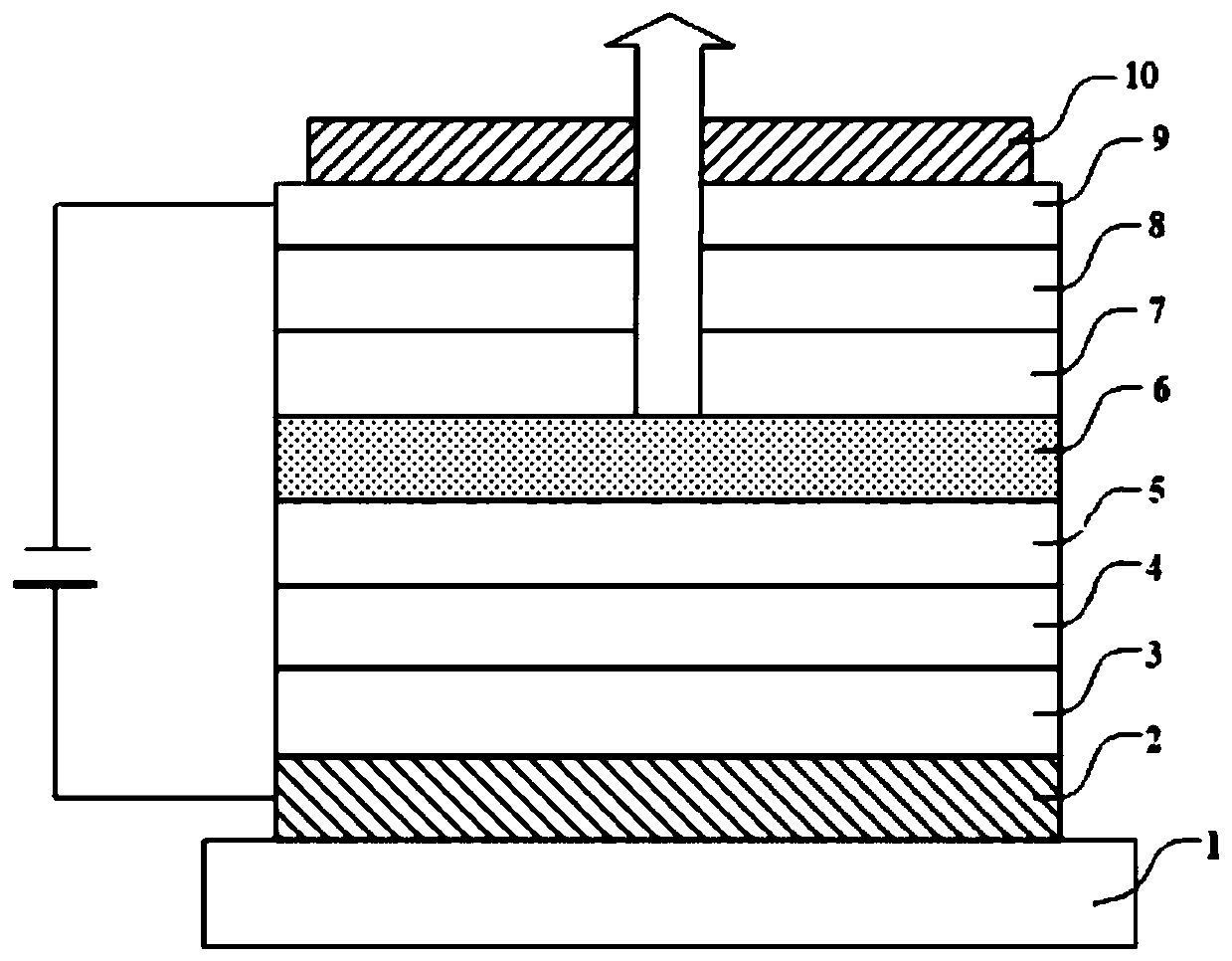
[0056] According to the nitrogen heterocyclic compound of the present invention, for visible light with a wavelength between 400nm and 700nm, the refractive index of the nitrogen heterocyclic compound is n≥2.0. The refractive index n≥2.0 meets the basic performance requirements of OLED devices for CPL materials, making it suitable for use as CPL materials.
[0057] According to the nitrogen heterocyclic compound of the present invention, for visible light with a wavelength of 430nm-600nm, the compound has an extinction coefficient k≤0.1. The wavelength of blue light is usually 400nm-450nm. The compound of the present invention has an extinction coefficient k≤0.1 for visible light with a wavelength of 430nm-6...
Embodiment 1
[0067] Synthesis of Compound P1
[0068] The synthetic route of compound P1 is as follows:
[0069]
[0070] (1) In a 250ml round bottom flask, add 1-1 (15mmol), diethyl malonate (35mmol) and sodium ethoxide (15mmol) into dry ethanol (100ml), under nitrogen atmosphere, 78°C The resulting mixed solution of the intermediate was added to water, then filtered through a celite pad, the filtrate was extracted with dichloromethane, washed with water, and dried with anhydrous magnesium sulfate. After filtration and evaporation, the The crude product was purified by column chromatography to obtain intermediate product 1-2.
[0071] (2) In a 250ml round bottom flask, add 1-2 (15mmol), diethylphenylamine (15mol) to dry POCl 3 (100ml), under a nitrogen atmosphere, reacted at 120°C for 6.0 hours, added the obtained intermediate mixed solution to water, then filtered through a diatomaceous earth pad, and the filtrate was extracted with dichloromethane, then washed with water, and used ...
Embodiment 2
[0077] Synthesis of Compound P17
[0078] The synthetic route of compound P17 is as follows:
[0079]
[0080] (1) In a 250ml round bottom flask, add 17-1 (15mmol), diethyl malonate (35mmol) and sodium ethoxide (15mmol) into dry ethanol (100ml), under nitrogen atmosphere, 78°C The resulting mixed solution of the intermediate was added to water, then filtered through a celite pad, the filtrate was extracted with dichloromethane, washed with water, and dried with anhydrous magnesium sulfate. After filtration and evaporation, the The crude product was purified by column chromatography to obtain intermediate product 17-2.
[0081] (2) In a 250ml round bottom flask, add 17-2 (15mmol), diethylphenylamine (15mol) to dry POCl 3 (100ml), under a nitrogen atmosphere, reacted at 120°C for 6.0 hours, added the obtained intermediate mixed solution to water, then filtered through a diatomaceous earth pad, and the filtrate was extracted with dichloromethane, then washed with water, and us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com