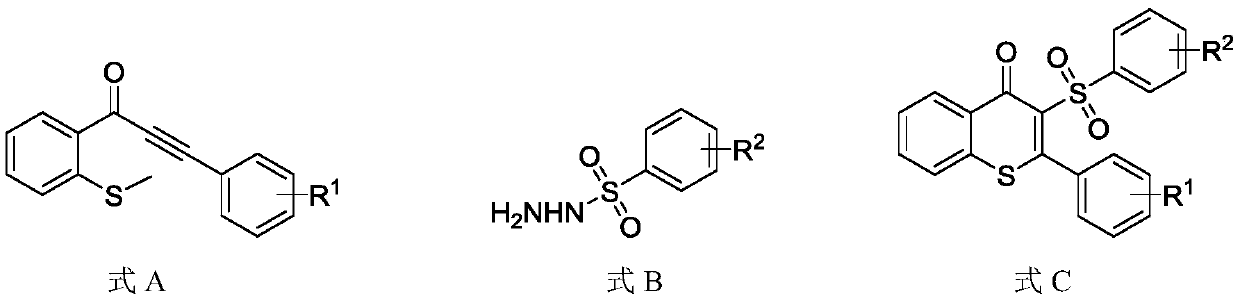
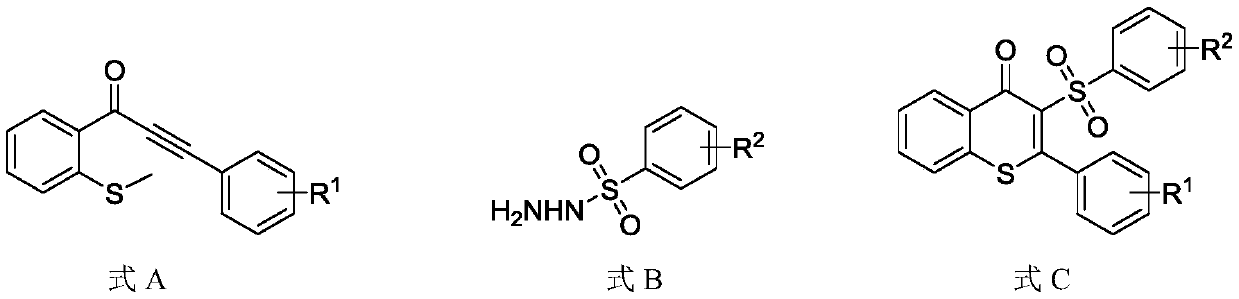
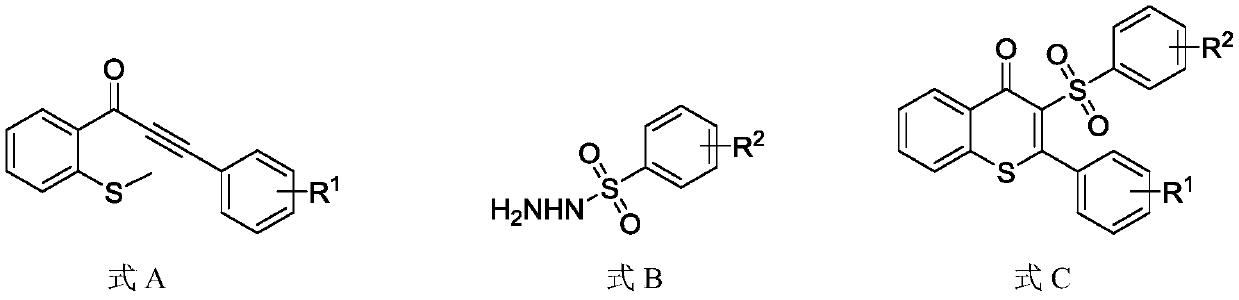
Synthesis method for synthesizing 3-sulfonylated thioflavonoid compound
A technology for sulfonylated sulfur and a synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as equipment corrosion and environmental pollution, and achieve the effects of mild reaction conditions, simple and safe operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 1-(2-(methylthio)phenyl)-3-phenyl-2-propynyl-1-one (0.2mmol) and phenylsulfonyl hydrazide to the 25mL reaction tube equipped with magnets. (0.4mmol), potassium persulfate (0.4mmol), catalyst 9-s-trimethyl-10-methylacridine perchlorate (0.006mmol); vacuum the reaction tube, replace it with nitrogen three times, and then add the solvent Ethylene glycol (2mL), the reaction tube was irradiated with a blue LED lamp with a wavelength of 460-465nm and stirred at a reaction temperature of 20°C for 12h. After the reaction, water was added to quench the reaction, the reaction solution was extracted with ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. The target product was obtained by column chromatography. The target product was a white solid with a melting point of 194-195°C. Based on the molar amount of 1-(2-(methylthio)phenyl)-3-phenyl-2-propynyl-1-one as 100%, the yield of the target product was 81%. The structural formula of ...
Embodiment 2
[0038] Add 1-(2-(methylthio)phenyl)-3-(4-methylphenyl)-2-propynyl-1-one (0.2mmol) to the 25mL reaction tube equipped with magnets. , Phenylsulfonyl hydrazide (0.2mmol), potassium persulfate (0.6mmol), catalyst 9-s-trimethyl-10-methylacridine perchlorate (0.002mmol); vacuum the reaction tube and use nitrogen Replace three times, then add solvent ethylene glycol (2mL), and stir the reaction tube for 12h at 10°C under blue light irradiation. After the completion of the reaction, water was added to quench the reaction, the reaction solution was extracted with ethyl acetate, the organic phases were combined and then dried over anhydrous sodium sulfate, and the target product was obtained by column chromatography. The target product was a white solid with a melting point of 150-152°C. Based on the molar amount of 1-(2-(methylthio)phenyl)-3-(4-methylphenyl)-2-propynyl-1-one as 100%, the yield of the target product is 85% . The structural formula of the target product is as follows: ...
Embodiment 3
[0045] Add 1-(2-(methylthio)phenyl)-3-(4-ethylphenyl)-2-propynyl-1-one (0.2mmol) to the 25mL reaction tube equipped with magnets. , Phenylsulfonyl hydrazide (0.4mmol), potassium persulfate (0.2mmol), catalyst 9-s-trimethyl-10-methylacridine perchlorate (0.01mmol); vacuum the reaction tube and use nitrogen It was replaced three times, and then the solvent ethylene glycol (2 mL) was added, and the reaction tube was stirred at 40° C. under blue light irradiation for 18 hours. After the reaction, water was added to quench the reaction, the reaction solution was extracted with ethyl acetate, and the organic phases were combined and dried with anhydrous sodium sulfate. The target product was obtained by column chromatography. The target product was a white solid with a melting point of 159-160 ℃. Based on the molar amount of 1-(2-(methylthio)phenyl)-3-(4-ethylphenyl)-2-propynyl-1-one as 100%, the yield of the target product is 80% , The structural formula of the target product is a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com