Synthetic method for preparing amide compounds through co-catalysis of niobium pentachloride and ionic liquid
A technology of amide compounds and ionic liquids, which is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem of low economy of reaction atoms, complicated post-treatment, Low yield and other problems, to achieve the effect of reducing catalyst consumption, less catalyst consumption, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
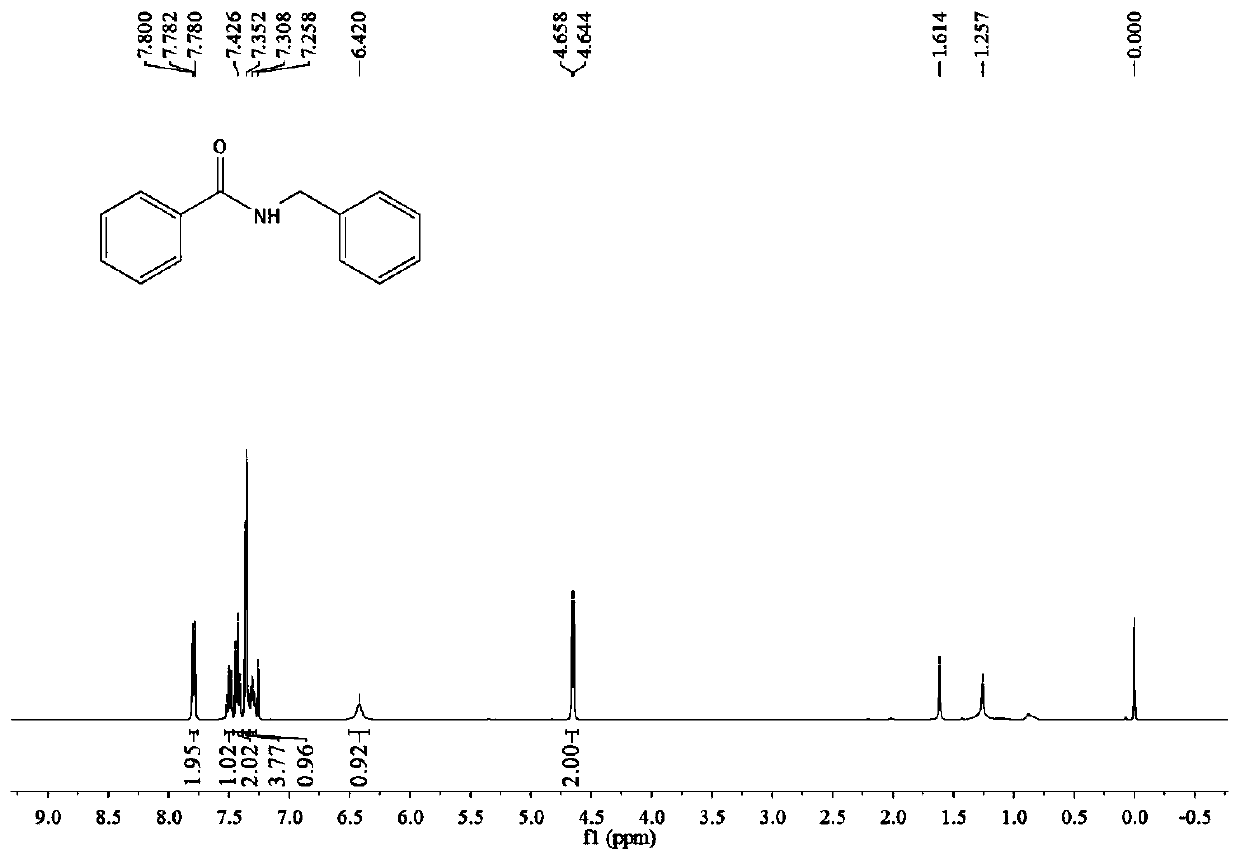
[0037] Benzoic acid (244mg, 2mmol), niobium pentachloride (11mg, 0.08mmol), ionic liquid 5 (22mg, 0.05mmol) and 4A molecular sieves (122mg, 50%, w / w%) were added to a round bottom flask, followed by Add toluene (4 mL, 2 mL / mmol), stir at room temperature for 30 minutes, then add benzylamine (214 mg, 2 mmol), heat up to reflux, and react for 24 hours. After the reaction was complete, the column was subjected to flash column chromatography (petroleum ether→ethyl acetate:triethylamine (200:1, v / v) to obtain 405 mg of a white solid with a yield of 96%. 1 H NMR (400MHz, CDCl 3 )δ7.809(d, J=7.2Hz, 2H), 7.50(t, J=7.2Hz, 1H), 7.43(t, J=7.2Hz, 2H), 7.38–7.34(m, 4H), 7.33– 7.27(m, 1H), 6.42(br.s, 1H), 4.65(d, J=5.6Hz, 1H), see figure 1 .
[0038] Synthesis of Ionic Liquid 5
[0039]
[0040] Add N-methylimidazole (4.6 g, 0.05 mol) into a round bottom flask and preheat it to 70° C., slowly add bromobutane dropwise, and continue heating to reflux for 4 h. After the com...
Embodiment 2
[0042]
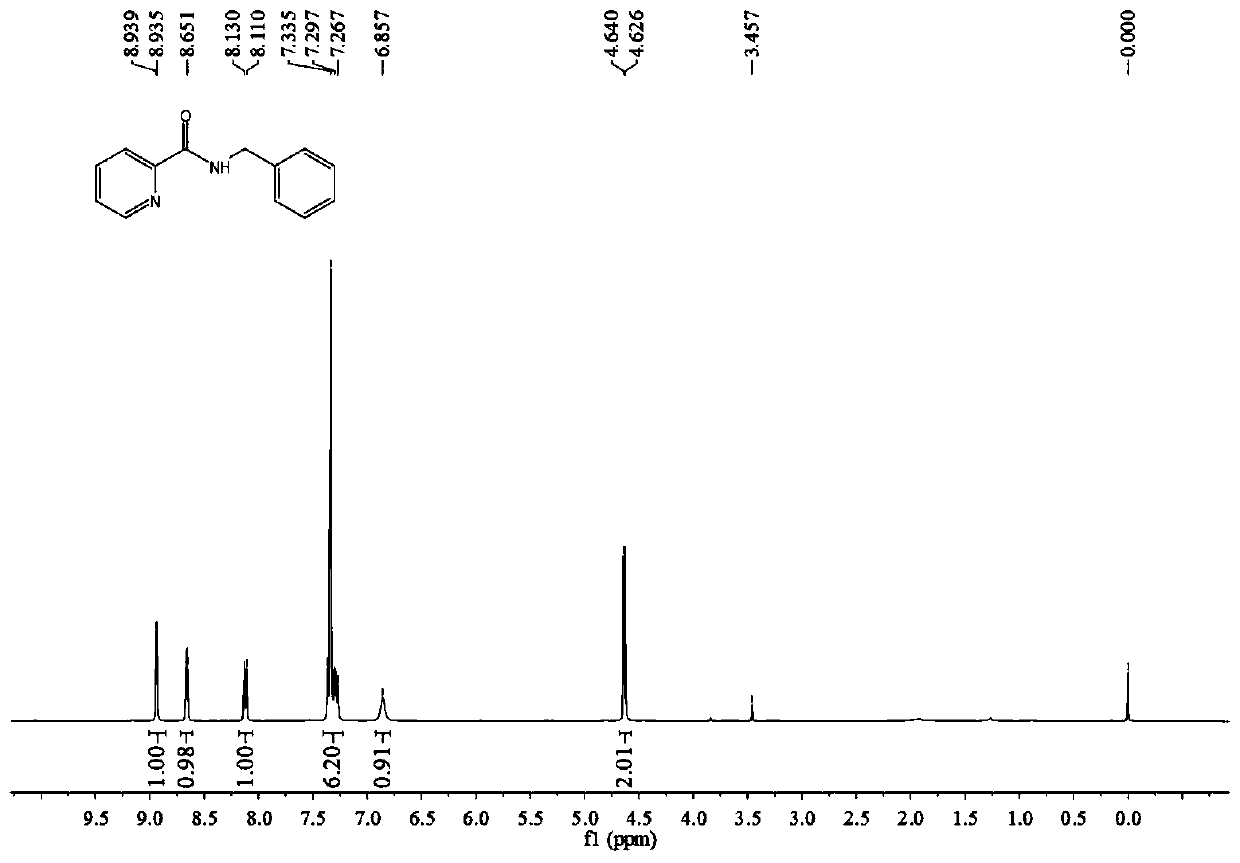
[0043] 2-Picolinic acid (246 mg, 2 mmol), niobium pentachloride (11 mg, 0.08 mmol), ionic liquid 5 (22 mg, 0.05 mmol) and 4A molecular sieves (122 mg, 50%, w / w%) were added to a round bottom flask , then added toluene (4mL, 2mL / mmol), stirred at room temperature for 30 minutes, then added benzylamine (214mg, 2mmol), raised the temperature to reflux, and reacted for 24 hours. After the reaction was complete, the column was subjected to flash column chromatography (petroleum ether→ethyl acetate:triethylamine (200:1, v / v) to obtain 365 mg of white solid with a yield of 86%. 1 H NMR (400MHz, CDCl 3 )δ8.94(d,J=1.9Hz,1H),8.66(dd,J=4.8,1.2Hz,1H),8.12(d,J=8.0Hz,1H),7.42–7.22(m,6H), 6.86(br.s, 1H), 4.63(d, J=5.7Hz, 2H), see figure 2 .
Embodiment 3
[0045]
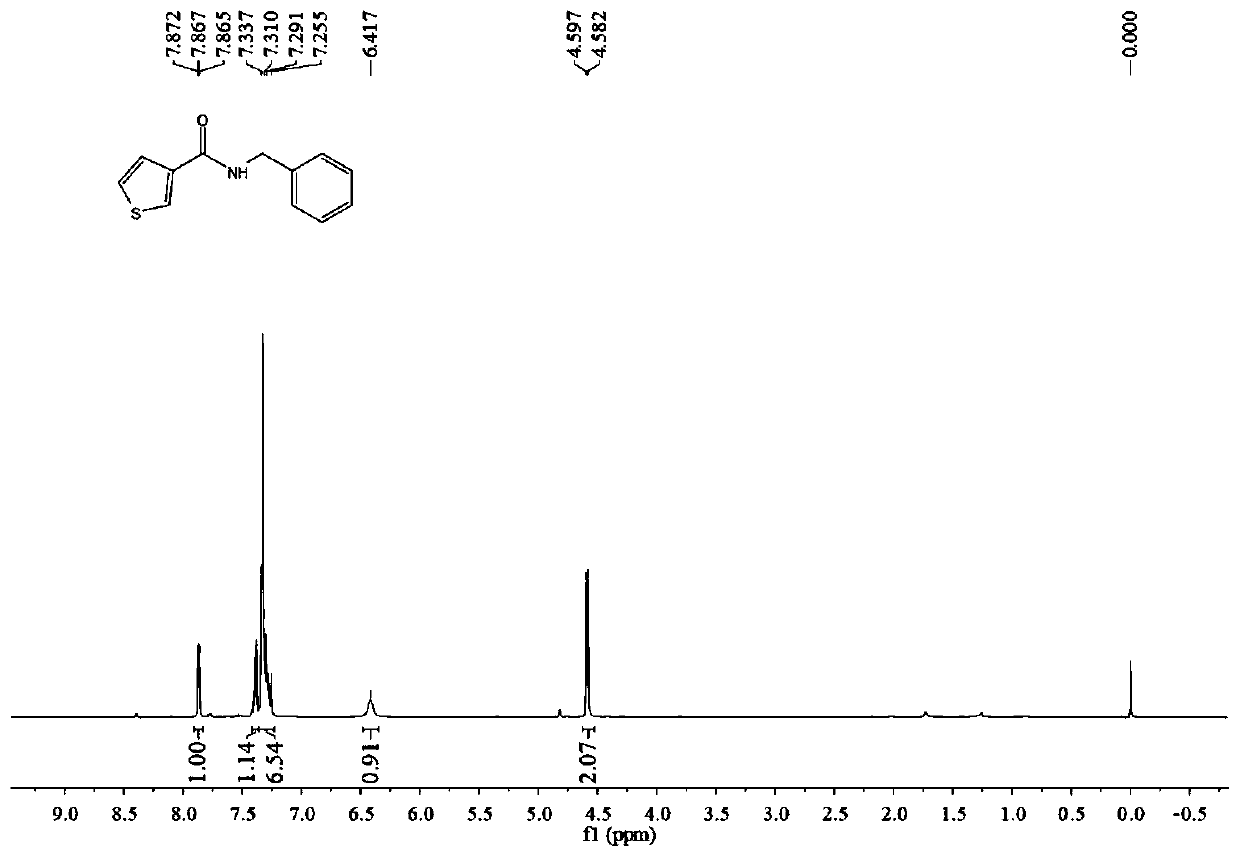
[0046] Add 3-thiophenecarboxylic acid (256 mg, 2 mmol), niobium pentachloride (11 mg, 0.08 mmol), ionic liquid 5 (22 mg, 0.05 mmol) and 4A molecular sieves (122 mg, 50%, w / w%) into a round bottom flask , then added toluene (4mL, 2mL / mmol), stirred at room temperature for 30 minutes, then added benzylamine (214mg, 2mmol), raised the temperature to reflux, and reacted for 24 hours. After the reaction was complete, the column was subjected to flash column chromatography (petroleum ether→ethyl acetate:triethylamine (200:1, v / v) to obtain 386 mg of white solid with a yield of 89%. 1 H NMR (400MHz, CDCl 3 )δ7.90–7.83(m,1H),7.39(dd,J=4.8,0.8Hz,1H),7.35–7.25(m,6H),6.42(br.s,1H),4.59(d,J= 6.0Hz, 2H), NMR see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com