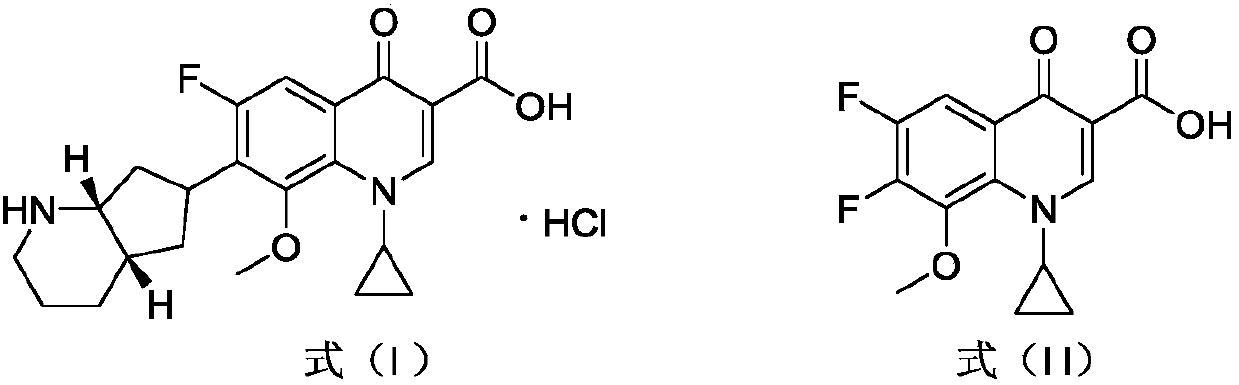
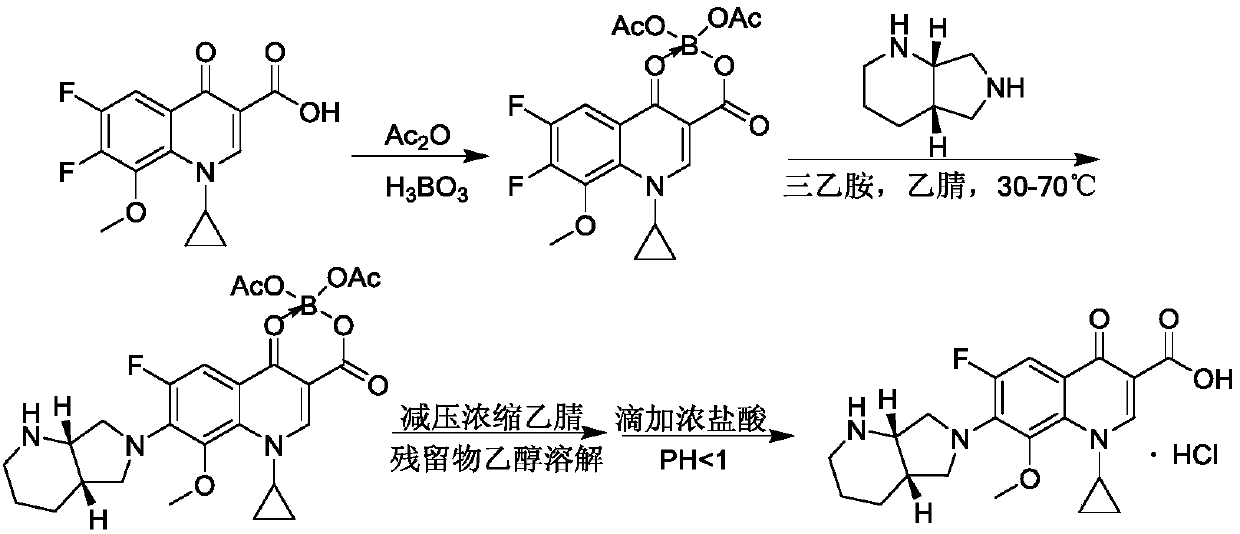
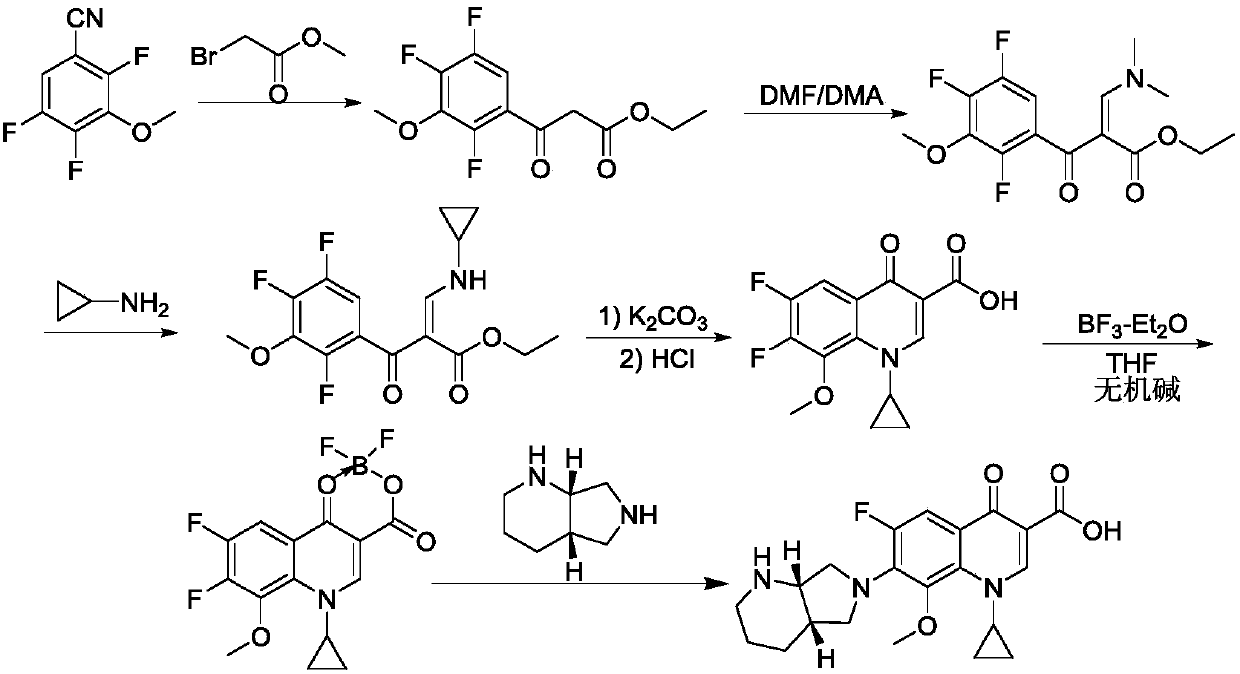
Synthetic method of moxifloxacin hydrochloride
A synthesis method and technology of moxifloxacin hydrochloride, applied in organic chemistry and other directions, can solve the problems of difficult industrialization, borate ester pollution, harsh reaction conditions, etc., and achieve the effects of eliminating environmental pollution, mild conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 8.850g gaticarboxylic acid in a 100mL two-necked flask, replace with argon, add 50mL isopropanol as solvent, then add 3.7857g (S,S)-2,8-diazabicyclo [4.3.0] Nonane, 4.2 mL of triethylamine and 4.2633 g of tetraisopropyl titanate. After the addition was complete, the temperature of the reaction liquid was raised to 100° C. for reaction. After the raw materials were completely consumed, the reaction was terminated, the reaction liquid was cooled to room temperature, transferred to a 100 mL single-necked flask, and the solvent isopropanol was distilled off under reduced pressure to obtain an oily substance. Add 100mL of 10% sodium hydroxide solution, shake, dissolve and continue to stir at 70°C for 2 hours, cool, filter, collect the filtrate, slowly adjust the pH of the filtrate to neutral conditions with dilute hydrochloric acid, and precipitate moxifloxacin monomer. Filter to collect moxifloxacin monomer.
[0039] Take another 250mL beaker, add moxifloxacin monom...
Embodiment 2
[0044]Weigh 8.850g of gaticarboxylic acid in a 100mL two-necked flask, replace with argon, add 50mL of ethanol as a solvent, and then add 3.7857g of (S,S)-2,8-diazabicyclo[4.3 .0] nonane, 4.2 mL triethylamine and 3.42 g tetraethyl titanate. After the addition was complete, the temperature of the reaction liquid was raised to 100° C. for reaction. After the raw materials were completely consumed, the reaction was terminated, the reaction liquid was cooled to room temperature, transferred to a 100 mL single-necked flask, and the solvent isopropanol was distilled off under reduced pressure to obtain an oily substance. Add 100mL of 10% sodium hydroxide solution, shake, dissolve and continue to stir at 70°C for 2 hours, cool, filter, and collect the filtrate. The filter cake is titanium solid waste, and the filtrate is slowly adjusted to a neutral condition with dilute hydrochloric acid. Moxifloxacin monomer was precipitated. Filter to collect moxifloxacin monomer.
[0045] Take...
Embodiment 3
[0047] Weigh 8.850g gaticarboxylic acid in a 100mL two-necked flask, replace with argon, add 50mL isopropanol as solvent, then add 3.7857g (S,S)-2,8-diazabicyclo [4.3.0] Nonane, 4.2 mL of triethylamine and 2.84 g of titanium tetrachloride. After the addition was complete, the temperature of the reaction liquid was raised to 100° C. for reaction. After the raw materials were completely consumed, the reaction was terminated, the reaction liquid was cooled to room temperature, transferred to a 100 mL single-necked flask, and the solvent isopropanol was distilled off under reduced pressure to obtain an oily substance. Add 100mL of 10% sodium hydroxide solution, shake, dissolve and continue to stir at 70°C for 2 hours, cool, filter, collect the filtrate, slowly adjust the pH of the filtrate to neutral conditions with dilute hydrochloric acid, and precipitate moxifloxacin monomer. Filter to collect moxifloxacin monomer.
[0048] Take another 250mL beaker, add moxifloxacin monomer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com