Nanobody GN2 composed of variable region of heavy chain antibody and preparation method and application of nanobody GN2
A nanobody and heavy chain antibody technology, applied in the biological field, can solve the problems of high production cost, strong immunogenicity, and large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Preparation of Nanobody GN2
[0075] Concrete preparation process comprises the following steps:
[0076] (1) Immune alpaca:
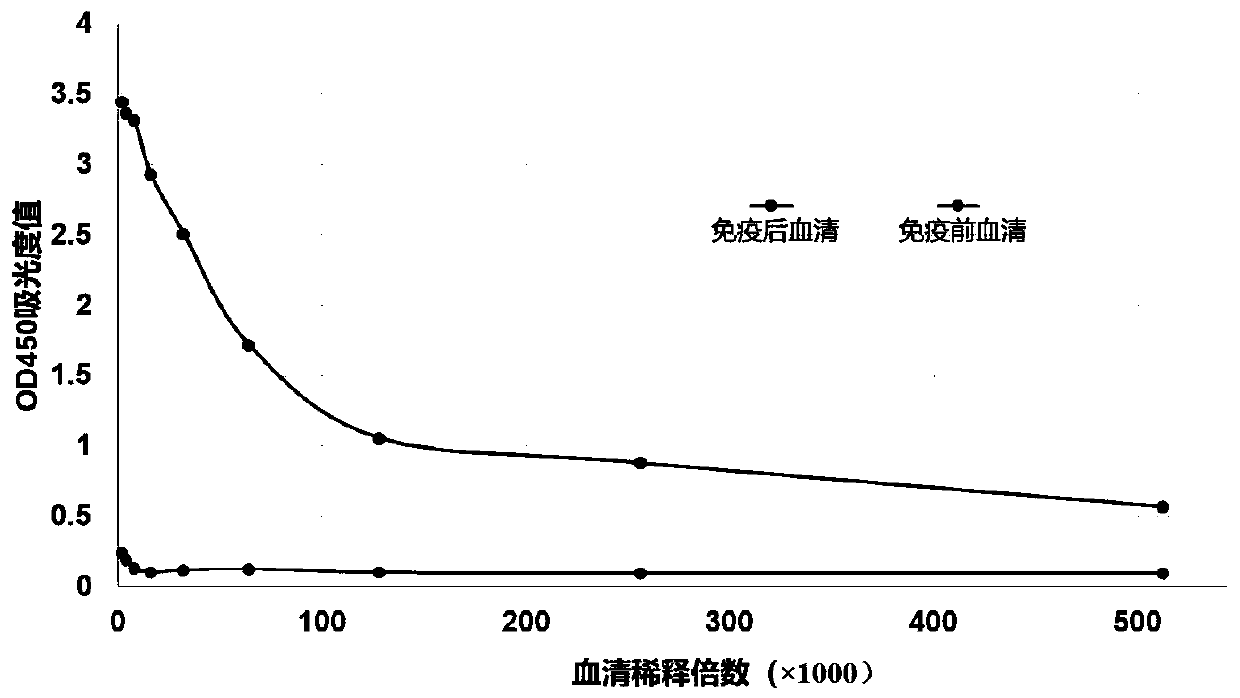
[0077] Take 1 mg of GPC3 protein expressed by eukaryotic HEK293 cells and emulsify with complete Freund's adjuvant, totaling 2 mL, and immunize a healthy adult alpaca for the first time by subcutaneous multi-point injection; on the 15th day, 0.5 mg of GPC3 protein Emulsified with Freund's complete adjuvant, a total of 2mL, subcutaneous multi-point injection for the second immunization; then use 0.5mg GPC3 protein emulsified with Freund's incomplete adjuvant to obtain a total of 2mL of immunization emulsified injection every 7 days for next immunization Immunity once. A total of 6 immunizations were performed, and blood was collected on the 7th day after each immunization to detect the titer. After testing the serum titer, 100 mL of peripheral blood was collected. The inventors found that the protein expressed by eukaryotic cells u...
Embodiment 2
[0104] Embodiment 2: nanobody GN2 thermal stability experiment:
[0105] Coat GPC3 protein on the ELISA well plate, 1 μg / mL GPC3 protein, add 100 μL per well to the microtiter plate, and coat overnight at 4°C. After the plate was washed three times with PBST, 300 μL of 5% skimmed milk was added to each well, and blocked for 1 hour at 37°C. Nanobody GN2 of the present invention and commercialized GPC3 monoclonal antibody (GPC3-mAb, Invitrogen) treated at different temperatures (4°C, 37°C, 60°C, 70°C, 80°C, 90°C) for 2 hours were added to each well. Add 100 μL to each well, incubate at room temperature for 1 hour, and wash the plate 3 times with PBST.
[0106] In the GN2 group, because the GN2 antibody has an HA tag, add HRP enzyme-labeled HA-mAb (SANTACRUZ company) to each well, incubate at room temperature for 40 minutes, wash the plate three times with PBST, add TMB for 10 minutes to develop color, and stop the reaction with 2M sulfuric acid. , the microplate reader detects...
Embodiment 3
[0108] Example 3: Establishment of a sandwich ELISA method for detecting serum GPC3 protein with GN2 nanobody-based GN2-luciferase fusion protein:
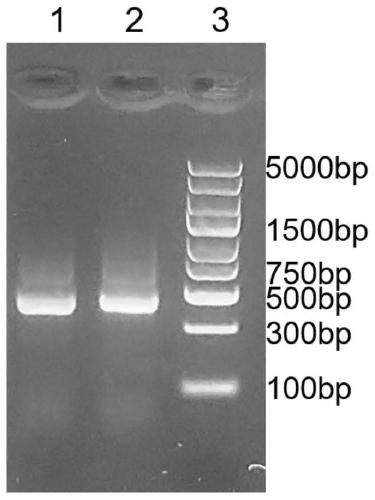
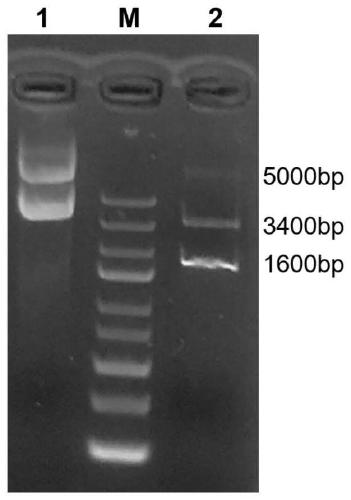
[0109] (1) Amplify the GN2 gene. Using the plasmid obtained in step (4) of Example 1 as a template, the GN2 nucleotide sequence was amplified by PCR; the PCR product was digested with Nco I and Sfi I, and the PCR product purification kit was used to purify and recover the digested product.
[0110] (2) Construction of fusion gene GN2-Luc. The synthetic fluorescent reporter gene is Nano-luciferase gene (Nano-luciferase, referred to as Luc) (the amino acid sequence of the fluorescent protein reporter gene luciferase is shown in SEQ ID NO.14): subcloned into the NotI and SalI sites of the vector pET22b Between, Nco I and Sfi I double digest the vector pET22b that contains fluorescent reporter gene nano-luciferase, cut the gel and reclaim the pET22b vector backbone sequence that contains luciferase gene; The vector backbone sequence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com