Application of esophageal cancer related antigen protein combination or specific antibody thereof in esophageal cancer detection kit
A technology for detecting kits and tumor-associated antigens, applied in the fields of molecular biology and oncology, to achieve the effects of screening and early detection, high detection accuracy, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
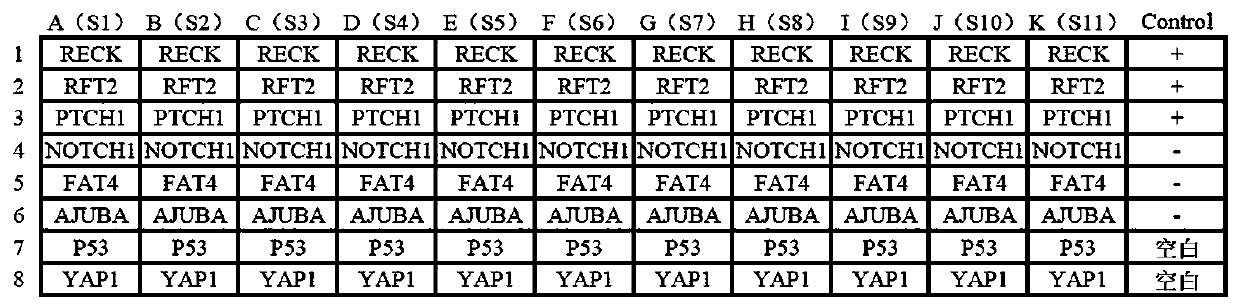
[0037] Example 1 Application of 8 kinds of tumor-associated antigens in the preparation of early screening esophageal cancer detection kit
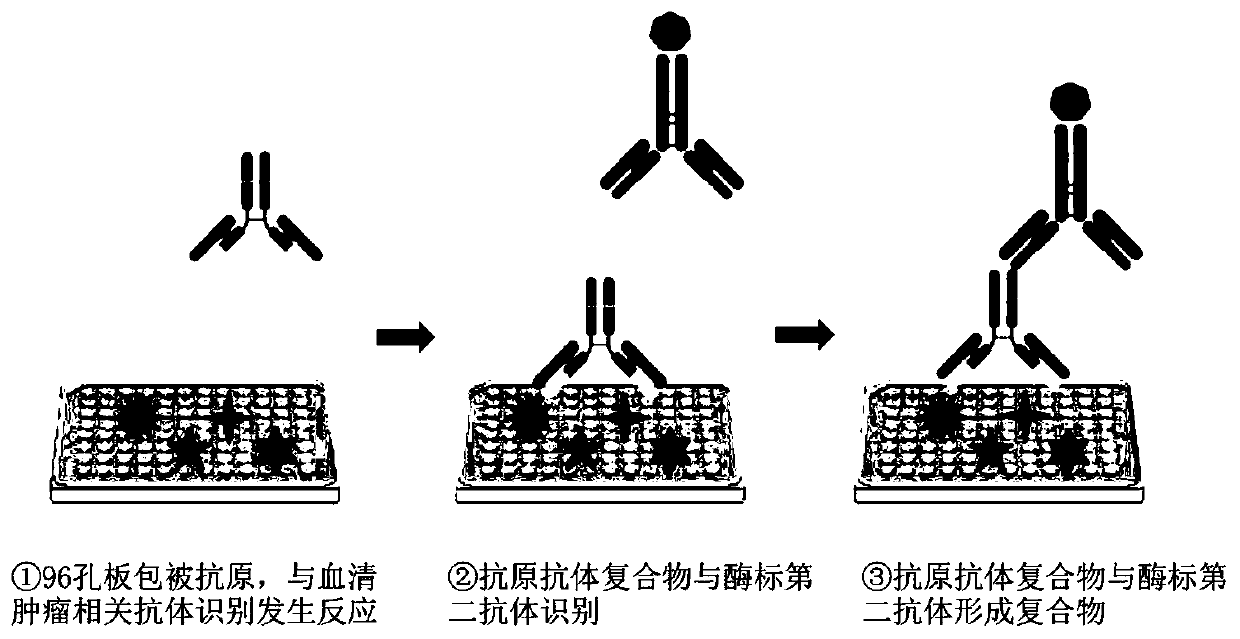
[0038] According to the principle of indirect enzyme-linked immunosorbent method, the present application prepares an autoantibody combined detection ELISA kit that can be used for screening and diagnosing early esophageal cancer. The principle of indirect enzyme-linked immunoassay figure 2 As shown, the antigen is connected to the solid-phase carrier, and the antibody to be tested in the sample is combined with it to form a solid-phase antigen-test antibody complex, and then the enzyme-labeled secondary antibody and the antibody in the solid-phase antigen-test antibody complex Combine to form a solid-phase antigen-test antibody-enzyme-labeled secondary antibody complex, and then measure the degree of color development after adding the substrate to determine the content of the antibody to be tested.
[0039] The ELISA kit used in this e...
Embodiment 2
[0040] The preparation of embodiment 2 ELISA kit
[0041] 1. Experimental materials and reagents:
[0042] (1) Eight tumor-associated antigen proteins (RECK, RFT2, PTCH1, NOTCH1, FAT4, AJUBA, P53 and YAP1);
[0043] (2) 96-well ELISA plate: 3590 (costar. USA);
[0044] (3) Coating solution: 50mM carbonate buffer solution, pH=9.6;
[0045] (4) Blocking solution: PBST buffer containing 2% (W / V) BSA;
[0046] (5) Sample diluent: PBST buffer containing 1% (W / V) BSA;
[0047] (6) Secondary antibody diluent: PBST buffer containing 1% (W / V) BSA;
[0048] (7) Enzyme-labeled secondary antibody: horseradish peroxidase-labeled RecA protein (Invitrogen);
[0049] (8) Washing solution: 0.01M PBST (phosphate) buffer at pH 7.4;
[0050] (9) Positive control serum: P53 positive control serum, that is, the serum of patients with esophageal cancer who were positive for P53 antibody by indirect ELISA and Western blot;
[0051] (10) Negative control serum: P53 negative control serum, that ...
Embodiment 3
[0072] The application process of embodiment 3 ELISA kit
[0073] 1. Sample source
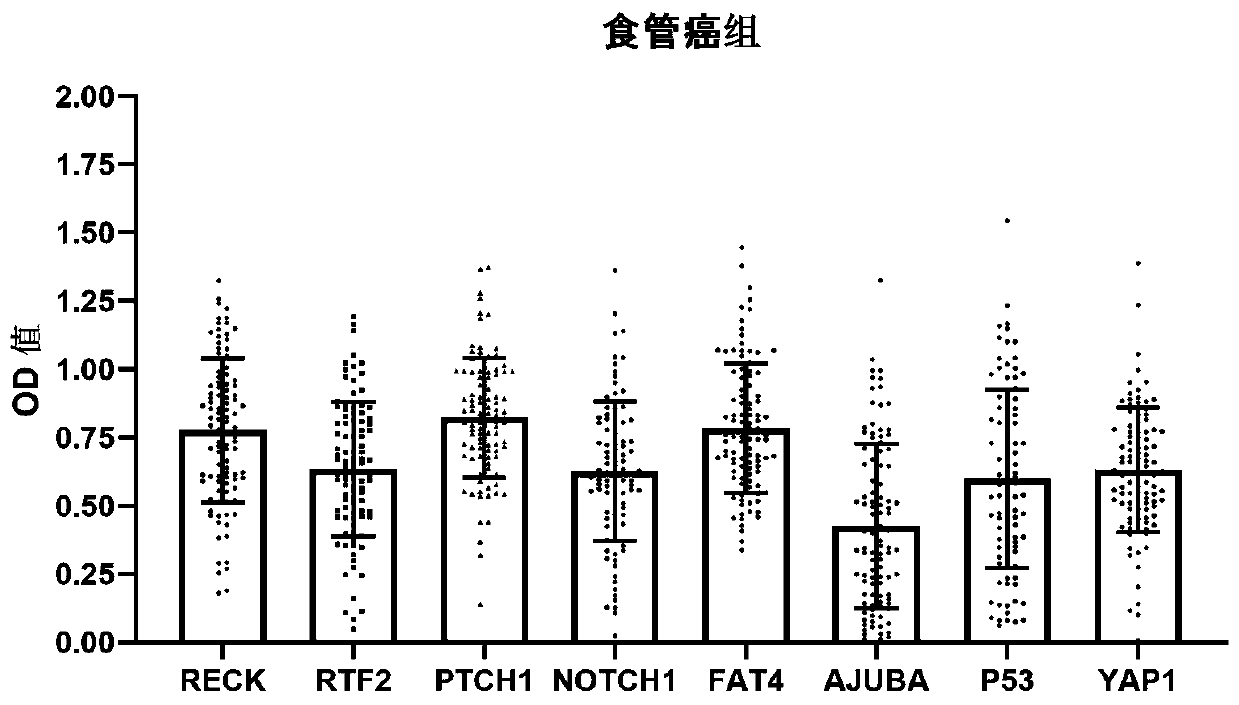
[0074] 400 serum samples were collected from the State Key Laboratory of Esophageal Cancer Prevention and Control jointly established by the province and ministry, including 200 serum samples from normal people (control group) and 200 serum samples from patients with early esophageal cancer (early esophageal cancer group). 200 cases of normal human serum were collected from the healthy checkup center of the laboratory's cooperative hospital, without any tumor-related evidence. Among the 200 normal subjects, there were 100 males and 100 females, the age range was 40-70 years old, and the average age was 58.9±5.3 years old. 200 serum samples from patients with early esophageal cancer were obtained from patients with early stage (stage 0+stage I) esophageal cancer confirmed by histopathology, and none of them received radiotherapy or chemotherapy. Among the 200 patients with esophageal cancer, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com