Hydrogen production catalyst by hydrolysis of sodium borohydride, preparation method and application thereof
A hydrogen production technology of sodium borohydride and hydrolysis, applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of easy agglomeration, shedding, oxidation, etc. , to achieve the effect of no environmental pollution, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of NiCoP / RC-malic acid catalyst
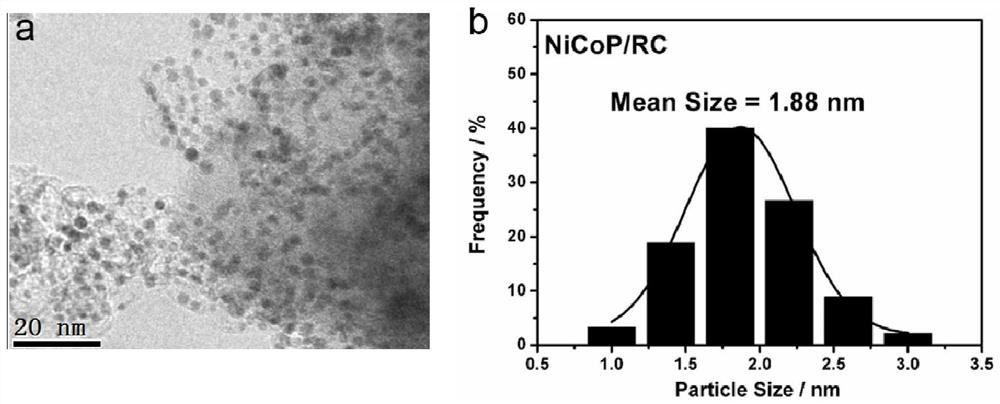
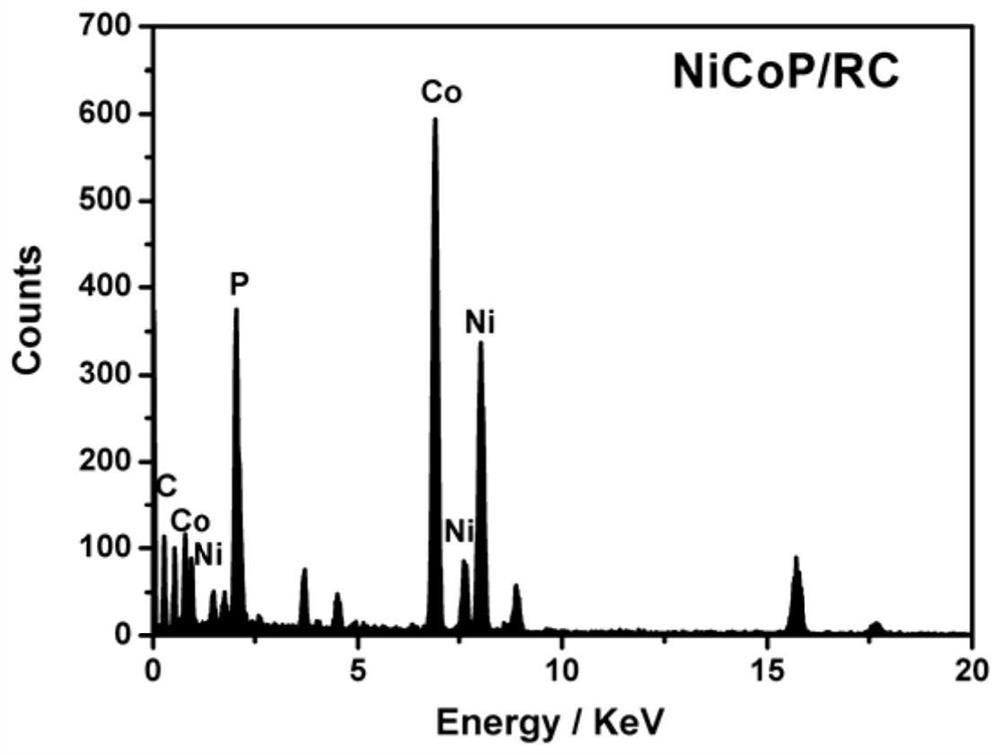
[0041] First, 20 g of phosphoramidate chelating resin was ball-milled for 3 hours to make it into powder. Subsequently, nickel chloride and cobalt chloride were dissolved in ultrapure water to prepare 0.15mol L -1 250mL of nickel chloride solution and 250mL of cobalt chloride solution were mixed together to form solution A; the ball-milled aminophosphoric acid chelating resin was added to solution A, and stirred at a speed of 750r / min for 24h; The resulting solution was then suction filtered under reduced pressure and washed with distilled water. Afterwards, the obtained solid was vacuum-dried at 50°C for 6h; and calcined in a tube furnace at 1000°C for 1h, under nitrogen protection during the calcination process, and the heating rate was 5°C / min to obtain NiCoP / RC.
[0042] The NiCoP / RC prepared above was mixed with malic acid at a mass ratio of 1:1 and ball milled for 3 hours to obtain a NiCoP / RC-malic acid mixed catalys...
Embodiment 2
[0049] Ni 2 Preparation of P / RC-citric acid catalyst
[0050] First, 20 g of phosphoramidate chelating resin was ball-milled for 3 hours to make it into powder. Subsequently, nickel chloride was dissolved in ultrapure water to prepare 0.05mol L -1 The solution. Next, add the phosphoramidate chelating resin after ball milling into 250mL of nickel chloride solution, and stir for 24h at a speed of 750r / min. The resulting solution was then suction filtered under reduced pressure and washed with distilled water. Afterwards, the obtained solid was vacuum-dried at 50° C. for 6 h. And calcined in a tube furnace at 600°C for 5h, under nitrogen protection during the calcining process, and the heating rate was 5°C / min, to obtain Ni 2 P / RC.
[0051] The above-prepared Ni 2 Mix P / RC and citric acid at a mass ratio of 1:0.5 and ball mill for 3 hours to obtain Ni 2 P / RC-citric acid mixed catalyst.
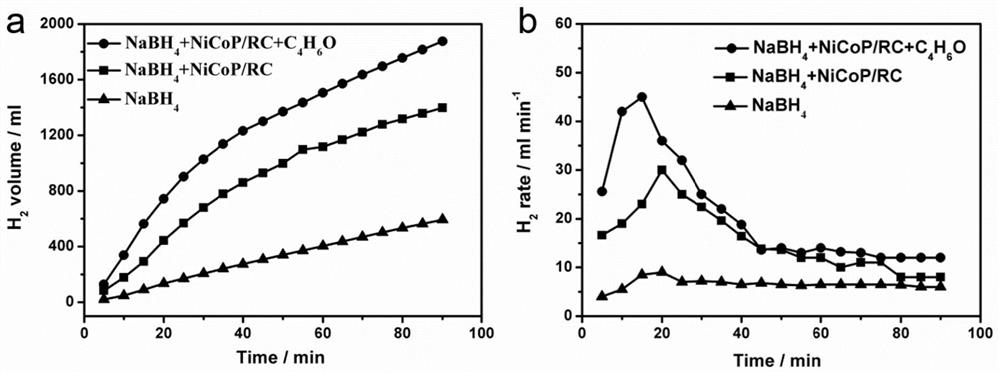
[0052] Subsequently, 1 g of sodium borohydride and 0.2 g of the prepared catalyst we...
Embodiment 3
[0055] co 2 Preparation of P / RC-phosphoric acid catalyst
[0056] First, 20 g of phosphoramidate chelating resin was ball-milled for 3 hours to make it into powder. Subsequently, cobalt nitrate was dissolved in ultrapure water to prepare 2.5mol L -1 The solution. Next, add the phosphoramidate chelating resin after ball milling into 500mL cobalt nitrate solution, and stir at a speed of 750r / min for 24h. The resulting solution was then suction filtered under reduced pressure and washed with distilled water. Afterwards, the obtained solid was vacuum-dried at 50° C. for 6 h. And calcined in a tube furnace at 1100°C for 3h, under nitrogen protection during the calcining process, and the heating rate was 10°C / min, that is, Co 2 P / RC.
[0057] The above-prepared Co 2 P / RC and oxalic acid were mixed at a mass ratio of 1:10 and ball milled for 3 hours to obtain a Co2P / RC-oxalic acid mixed catalyst.
[0058] Subsequently, 1 g of sodium borohydride and 0.2 g of the prepared catal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com