Supported heteropolyacid catalyst and preparation method thereof
A technology of heteropolyacid and catalyst, applied in the field of supported heteropolyacid catalyst and its preparation, can solve the problems of poor low temperature activity, high equipment requirements and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
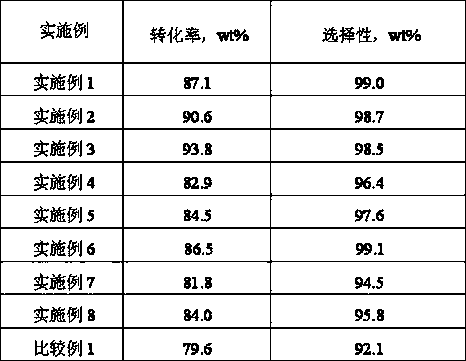
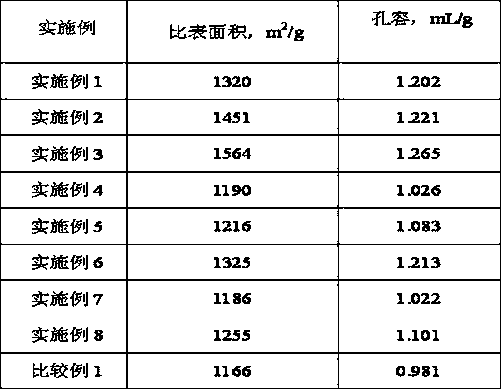
Embodiment 1
[0041] (1) Catalyst preparation
[0042] Weigh 600g of ammonium phosphate, dissolve it in 2000mL of deionized water to obtain solution A; grind 1000g of petroleum coke to powder, then add it to solution A, let it stand for 1.5h, then filter, and put the obtained solid sample in an oven at 110°C Dry for 5h. At 200°C, pretreat the dried solid sample with water vapor for 3 hours (the volumetric space velocity of the water vapor gas is 1200 hours -1 ), then raise the temperature to 400°C, continue pretreatment for 3h, and then cool to 60°C under nitrogen protection to obtain pretreated petroleum coke.
[0043] Mix 1000g of the pretreated petroleum coke obtained above, 38.5g of lanthanum nitrate and 2000g of potassium hydroxide evenly, place in a microwave heating furnace with a microwave frequency of 2450MHz, vacuumize, and heat up to 500 under the condition that the microwave power is 0.3kw ℃, keep it constant for 40min, then pass nitrogen gas to normal pressure, and continue t...
Embodiment 2
[0049] (1) Catalyst preparation
[0050] Weigh 500g of diammonium hydrogen phosphate, dissolve it in 2000mL of deionized water to obtain solution A; grind 1000g of petroleum coke to powder, then add it to solution A, leave it for 1.5h, then filter, and put the obtained solid sample in an oven at 110 Dry at ℃ for 5h. At 200°C, use water vapor to pretreat the dried solid sample for 3 hours (the volumetric space velocity of the water vapor gas is 1000h -1 ), then raise the temperature to 400°C, continue pretreatment for 3h, and then cool to 60°C under nitrogen protection to obtain pretreated petroleum coke.
[0051] Mix 1000g of the pretreated petroleum coke obtained above, 62.2g of lanthanum nitrate and 3000g of potassium hydroxide evenly, place in a microwave heating furnace with a microwave frequency of 2450MHz, vacuumize, and heat up to 600 ℃, keep it constant for 20min, then pass nitrogen gas to normal pressure, continue to raise the temperature to 900℃ for 10min under the...
Embodiment 3
[0057] (1) Catalyst preparation
[0058] Weigh 400g of ammonium dihydrogen phosphate, dissolve it in 2000mL deionized water to obtain solution A; grind 1000g of petroleum coke to powder, then add it to solution A, leave it for 1.5h, then filter, and put the obtained solid sample in an oven at 110 Dry at ℃ for 5h. At 200°C, pretreat the dried solid sample with water vapor for 3 hours (the volumetric space velocity of the water vapor gas is 800 hours) -1 ), then raise the temperature to 400°C, continue pretreatment for 3h, and then cool to 60°C under nitrogen protection to obtain pretreated petroleum coke.
[0059] Mix 1000g of the pretreated petroleum coke obtained above, 89.9g of lanthanum nitrate and 4000g of potassium hydroxide evenly, place in a microwave heating furnace with a microwave frequency of 2450MHz, vacuumize, and heat up to 400 under the condition that the microwave power is 0.3kw ℃, keep it constant for 60min, then pass nitrogen gas to normal pressure, continu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com