Radix stemonae alkaloid analogue near-infrared fluorescent probe and preparation method thereof
A fluorescent probe and near-infrared technology, applied in the field of optical imaging, can solve the problems of short emission wavelength, complex synthesis route, and long response time, and achieve the effects of rapid fluorescence detection, easy availability of raw materials, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: The specific synthetic process of fluorescent probe of the present invention
[0028] (1) The specific synthetic steps of compound 2 are as follows:
[0029]
[0030] 2,6-bis(hydroxymethyl)-p-cresol (5g, 29mmol) and MnO 2 (20 g, 229 mmol) in 400 ml of acetone was stirred overnight at room temperature, filtered and the filtrate was concentrated to give a residue. Separation by column chromatography (PE:EA=2:1) gave compound 5 (53%) as a white solid.
[0031]
[0032] Compound 5 (278 mg, 1.67 mmol), cyclopent-2-en-1-one (compound 6) (0.280 mL, 3.35 mmol) and 1-H-imidazole (120 mg, 1.67 mmol) were dissolved in tetrahydrofuran (THF, 2.5 mL) and distilled water (2.5mL). The mixture was stirred at room temperature for 96 h, the final mixture was diluted with 10 mL of HCl solution (1 mol / L), extracted with ethyl acetate (3×15.27 mL). The resulting residue was concentrated from the filtrate and separated by silica gel column chromatography (PE:EA=1:2) ...
Embodiment 2
[0036] Embodiment 2. The concrete synthetic steps of fluorescent probe are as follows:
[0037]
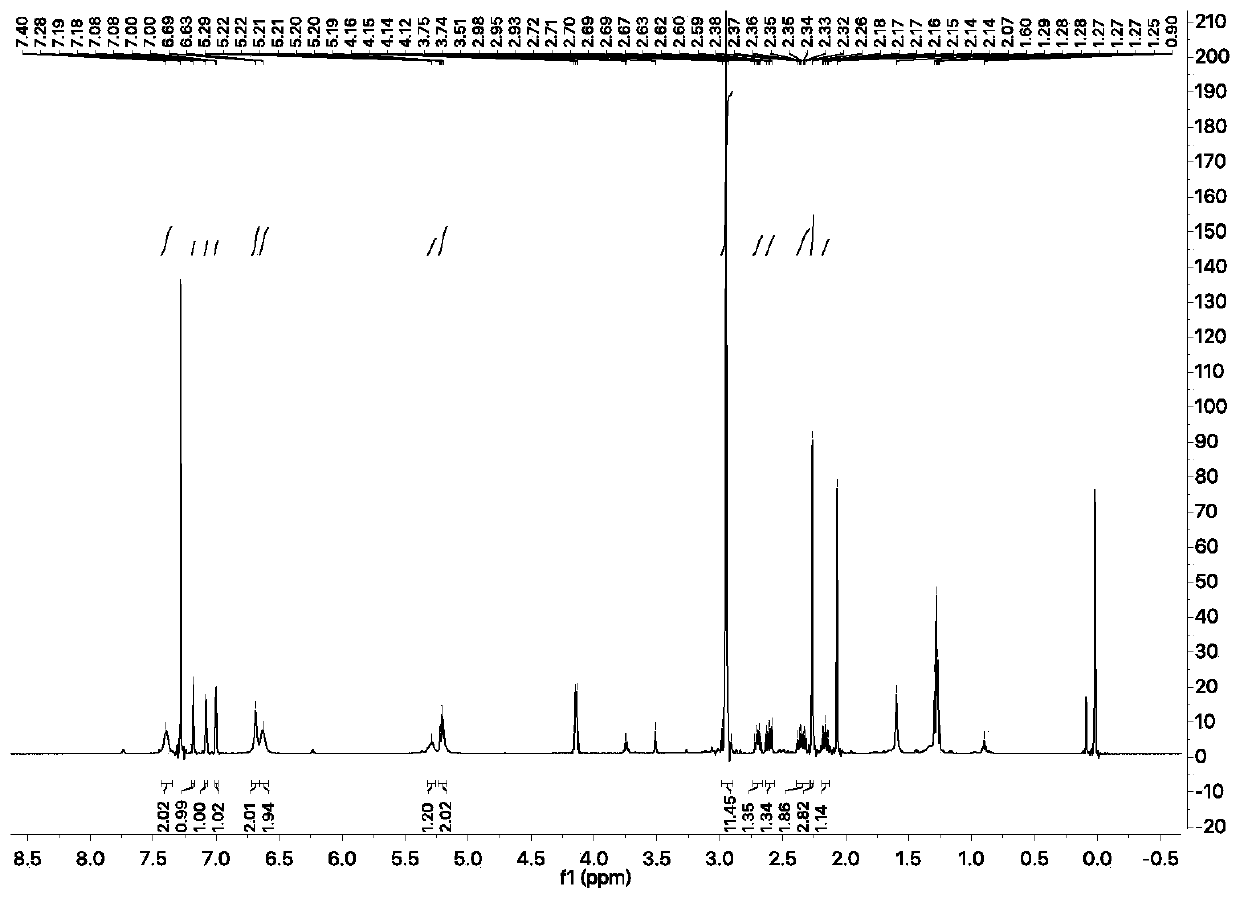
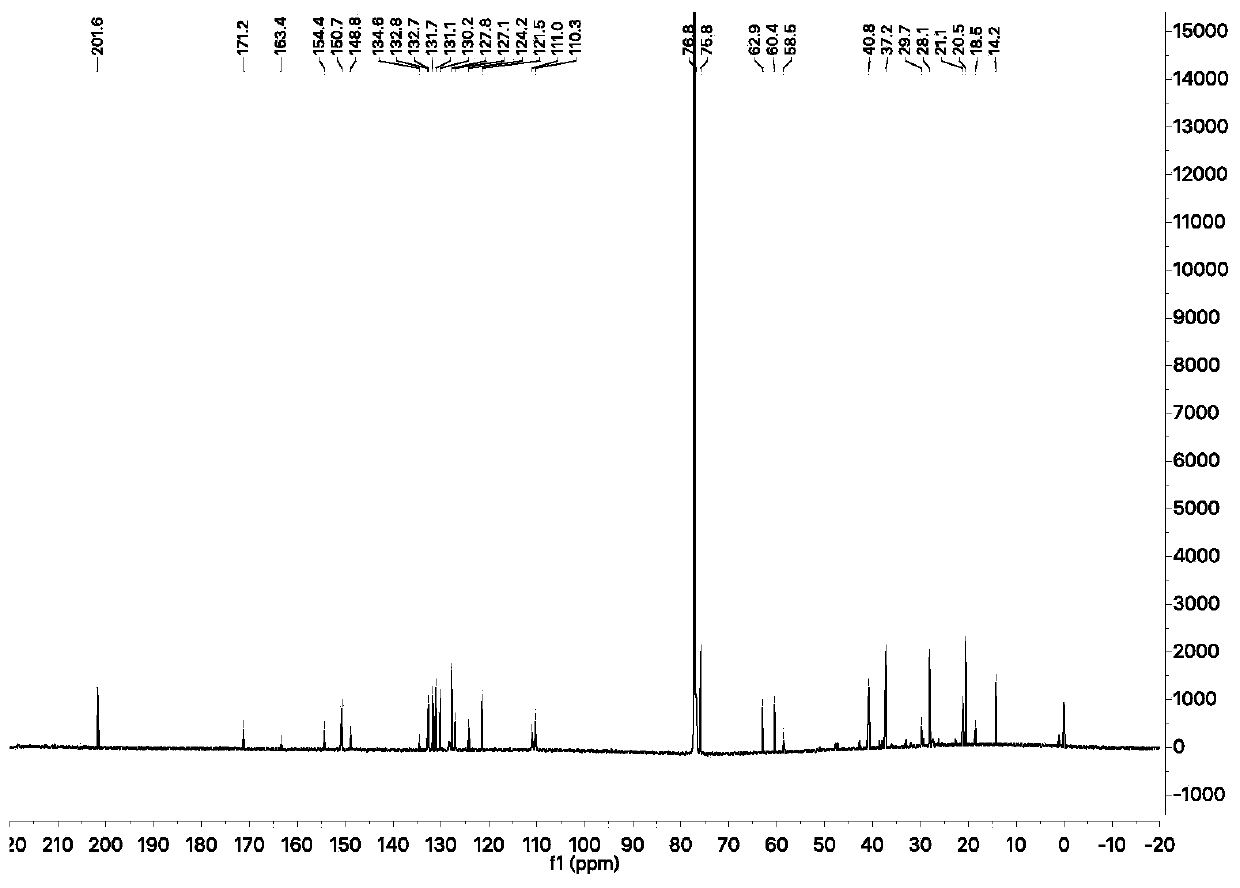
[0038] Compound 2 (32 mg, 139 μmol, 1 eq), compound 3 (58 mg, 167 μmol, 1.2 eq) and DMAP (57 mg, 470 μmol, 3.38 eq) were dissolved in 0.64 mL of pyridine and stirred overnight at 45 °C under argon. The reaction was monitored by TLC spot plate until the reaction of the raw materials was completed, the reaction solution was precipitated with diethyl ether, and the diethyl ether layer was dried, the filtrate was concentrated to obtain the residue, and separated by silica gel column chromatography (PE:EA=2:1, 5% triethylamine) Obtained 49 mg of fluorescent probe. 1 H NMR (600MHz, CDCl 3 )δ7.40(s,2H),7.18(d,J=2.5Hz,1H),7.08(d,J=2.2Hz,1H),7.00(d,J=2.2Hz,1H),6.69(s, 2H), 6.63(s, 2H), 5.29(s, 1H), 5.21(ddd, J=9.6, 7.5, 2.5Hz, 2H), 2.95(s, 12H), 2.70(dt, J=12.3, 8.1Hz ,1H),2.61(dd,J=18.5,9.0Hz,1H),2.35(ddd,J=18.6,12.7,8.7Hz,1H),2.26(s,3H),2.16(ddd,J=12.5,9.1 ,3.5Hz,1H). 13 C NMR (1...
Embodiment 3
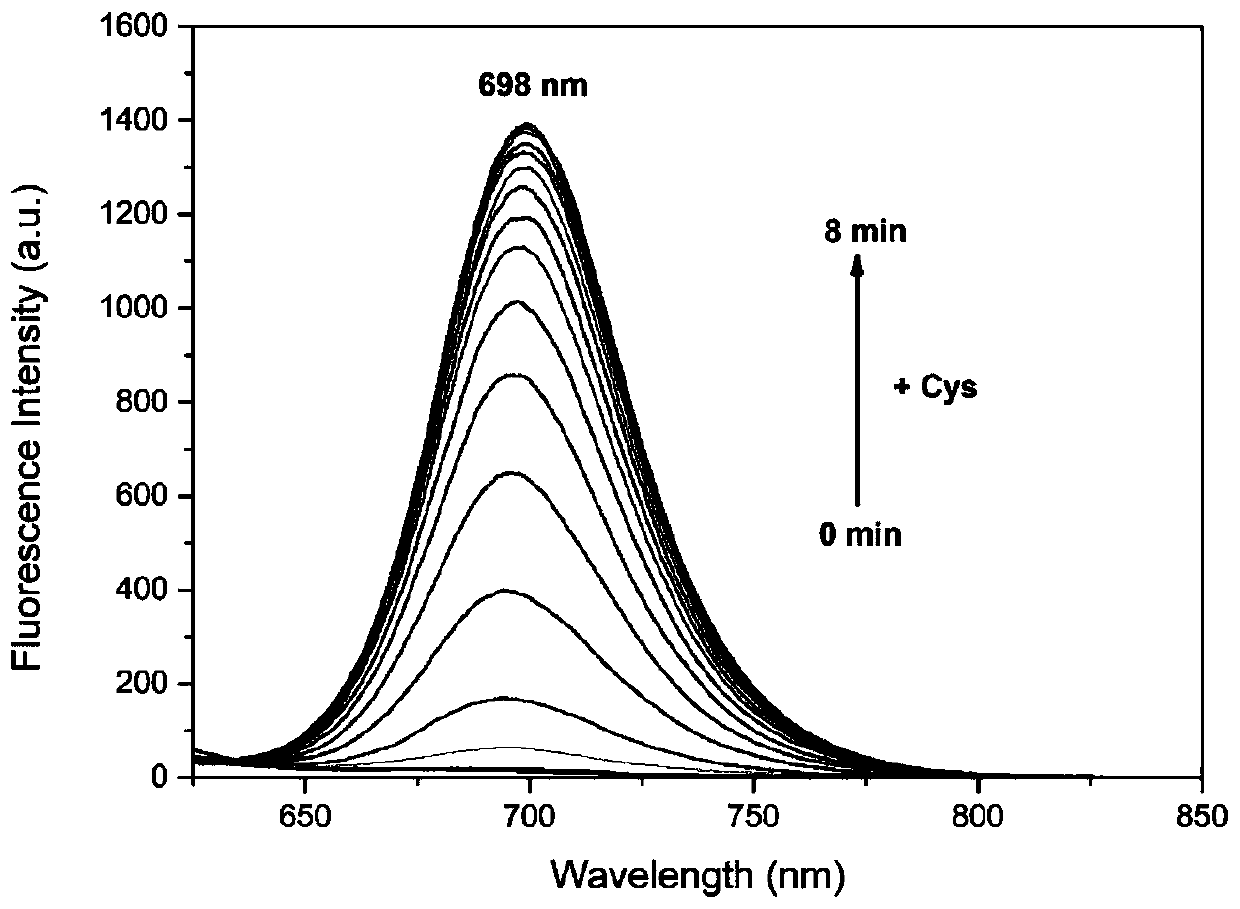
[0039] Embodiment 3: before and after the reaction of the fluorescent probe of the present invention and Cys, the ultraviolet and fluorescence spectrograms:
[0040] Add 140 μM Cys (stirring) to the fluorescent probe solution of the present invention (10 μM, PBS:DMSO=1:1), and measure the changes of ultraviolet and fluorescence intensity after 10 min. The results can be found in image 3 with Figure 4 , all UV and fluorescence intensities were normalized.
[0041] Such as image 3As shown, the pictures of color changes visible to the naked eye without adding (left) and adding (right) Cys to the fluorescent probe solution of the present invention. After adding Cys, the fluorescence changes obviously, from colorless to blue, indicating that the probe reacts with Cys and can be used to trace Cys.
[0042] Such as image 3 Shown is the ultraviolet-visible absorption spectrum of the fluorescent probe solution of the present invention before and after adding Cys. It can be fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com