Sodium ion battery positive electrode material, preparation method thereof and sodium ion battery
A sodium-ion battery and positive electrode material technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve problems such as poor stability, large side reactions between electrode materials and electrolyte, and low charge and discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
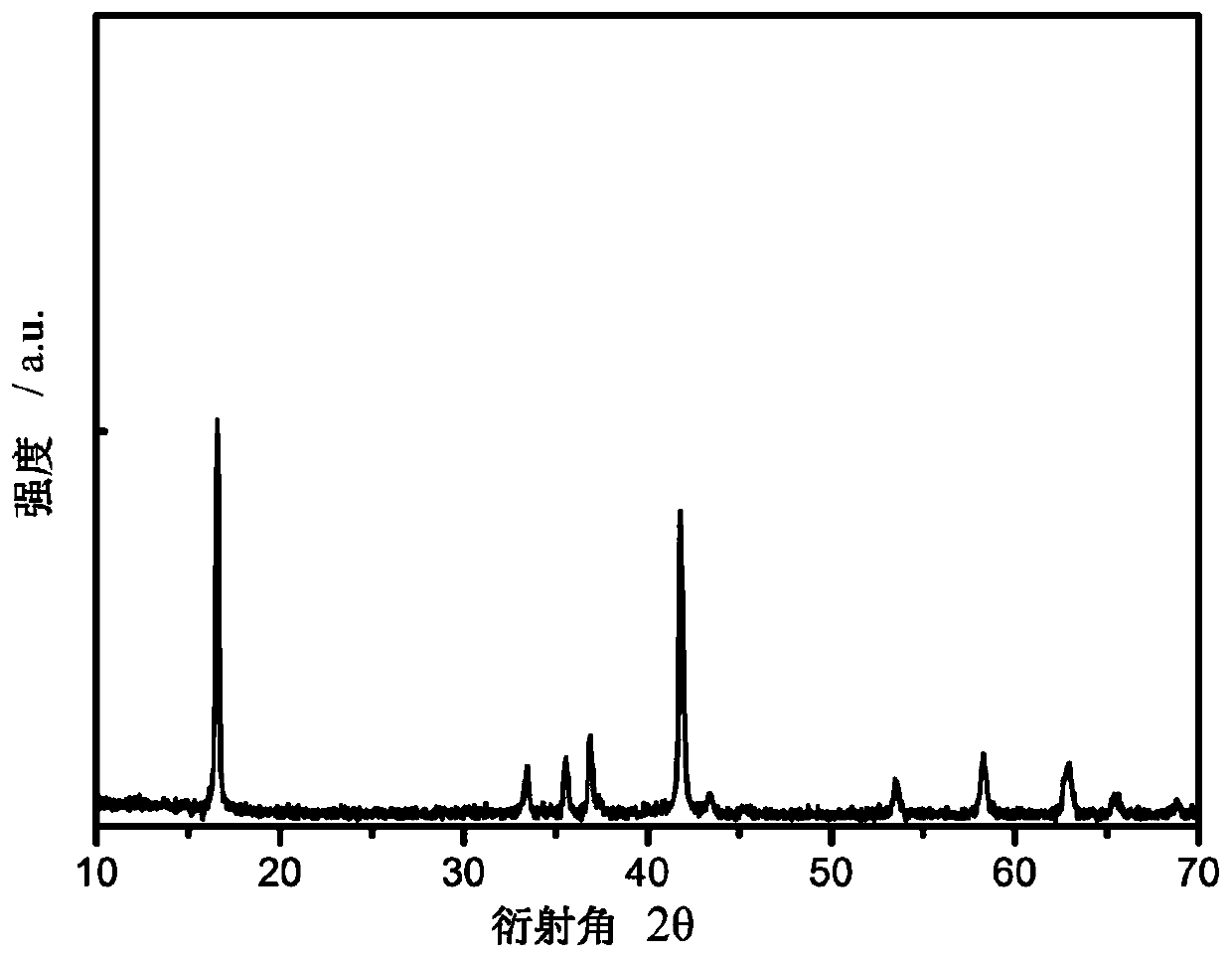
Image
Examples
Embodiment 1
[0062] (1) Preparation of layered compounds
[0063] Weigh ferrous sulfate, manganese sulfate, nickel sulfate, and titanyl sulfate in sequence so that the molar ratio of iron, manganese, nickel, and titanium is 0.6:0.1:0.2:0.1 to prepare a soluble transition metal salt. Using deionized water as a solvent, the above-mentioned soluble transition metal salt is formulated into a mixed salt solution with a concentration of 2mol / L, sodium hydroxide is formulated with a precipitant with a concentration of 5mol / L, and ammonia water is formulated with a concentration of 5mol / L. Complexing agent. Add the mixed salt solution, precipitating agent and complexing agent into the reaction kettle at the same time. After the co-precipitation reaction, let it stand for 12 hours, filter, wash with deionized water twice, and dry at 100°C for 10 hours to obtain the precursor powder. After uniformly mixing the above precursor powder with sodium carbonate, sintering at 900°C for 15 hours in an air a...
Embodiment 2
[0067] (1) Preparation of layered compounds
[0068] Weigh ferrous sulfate, manganese sulfate, nickel sulfate, titanyl sulfate, and copper sulfate in sequence so that the molar ratio of iron, manganese, nickel, titanium, and copper is 0.5:0.1:0.2:0.1:0.1 to make a soluble transition metal salt. Using deionized water as a solvent, the above-mentioned soluble transition metal salt is formulated into a mixed salt solution with a concentration of 1mol / L, sodium hydroxide is formulated with a precipitant with a concentration of 2mol / L, and ammonia water is formulated with a concentration of 2mol / L. Complexing agent. The mixed salt solution, precipitating agent and complexing agent were added into the reaction kettle at the same time and co-precipitated. After the co-precipitation reaction, it was aged for 15 hours, filtered, washed twice with deionized water, and dried at 120°C for 12 hours to obtain the precursor powder. After uniformly mixing the above precursor powder with sodi...
Embodiment 3
[0072] (1) Preparation of layered compounds
[0073] Weigh ferrous nitrate, manganese nitrate, nickel nitrate, copper sulfate, and tin tetrachloride in turn, so that the molar ratio of iron, manganese, nickel, copper, and tin is 0.5:0.2:0.1:0.1:0.1 to make a soluble transition metal salt . Using deionized water as a solvent, the above-mentioned soluble transition metal salt is formulated into a mixed salt solution with a concentration of 1mol / L, sodium hydroxide is formulated into a precipitant with a concentration of 4mol / L, and ammonia water is formulated into a concentration of 4mol / L. Complexing agent. Add the mixed salt solution, precipitating agent and complexing agent into the reaction kettle at the same time. After the co-precipitation reaction, let it stand for 10 hours, filter, wash with deionized water twice, and dry at 110°C for 10 hours to obtain the precursor powder. After uniformly mixing the above precursor powder with sodium carbonate, sintering at 800°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com