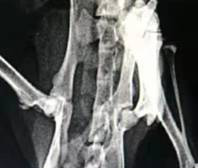
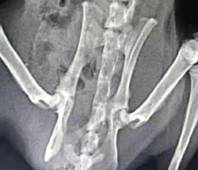
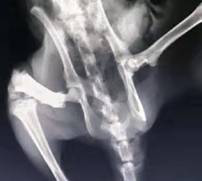
A compound acellular matrix injection for treating femoral head necrosis and using method
A technology of acellular matrix and femoral head necrosis, which is applied in drug combinations, pharmaceutical formulas, bone diseases, etc., can solve the problems of inability to fix mesenchymal stem cells, inability to provide a regenerative microenvironment, and unfavorable renal excretion, so as to avoid pioneering surgery risk, speed up the full recovery time, and the effect of no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the preparation method of composite acellular matrix injection
[0054] Preparation method of acellular matrix solution: (1) Firstly weigh 500g of porcine Achilles tendon tissue, wash it with PBS and other pretreatments, inactivate the virus and crush it; (2) Add 2L of 10% sodium chloride solution for soaking Shaking table for 2 hours, 30°C / 200rpm; (3) filter, wash the tissue with a large amount of purified water 3 times, 30min each time, shaker for 10min, 30°C / 200rpm; (4) use 2L 3% sodium carbonate Soak the solution for 3 hours to degrease, wash with purified water 3 times: 10min / time, 30℃ / 200rpm; (5) Soak the shaker with 5L 4% trypsin for 3 hours, wash with purified water 3 times: 10min / time, 30℃ / 200rpm; ( 6) Degrade the nucleic acid components in the cells by nuclease, wash thoroughly to remove the detergent to obtain the acellular matrix, and freeze-dry to obtain the acellular matrix powder; (7) prepare pH=2 HCl solution, 10×PBS solution, 1×PBS soluti...
Embodiment 2
[0058] Example 2. Take 1.2 parts of acellular matrix liquid, 1 part of bone marrow mesenchymal stem cell liquid and 0.8 part of Angelica Jisheng injection in the composite acellular matrix injection in Example 1, and mix them according to the proportion during the operation. Fill into a 10ml syringe and compound into an injectable mixture.
Embodiment 3
[0059] Example 3. Take 0.8 parts of acellular matrix liquid, 1 part of bone marrow mesenchymal stem cell liquid and 1.2 parts of Angelica Jisheng injection in the composite acellular matrix injection in Example 1, and mix them according to the proportion during the operation. Fill into a 10ml syringe and compound into an injectable mixture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com