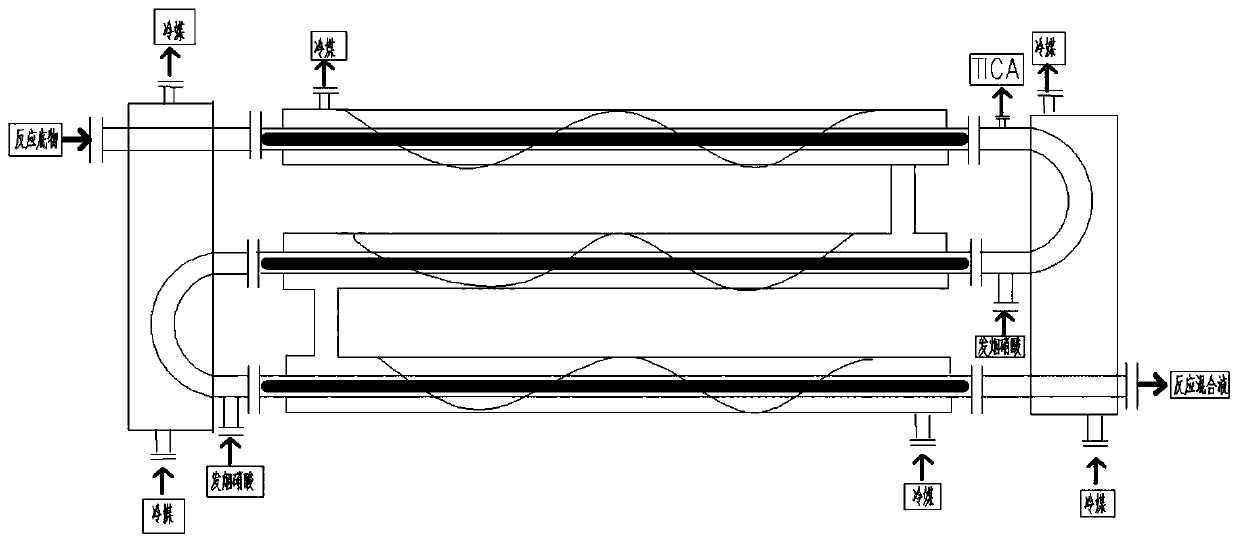
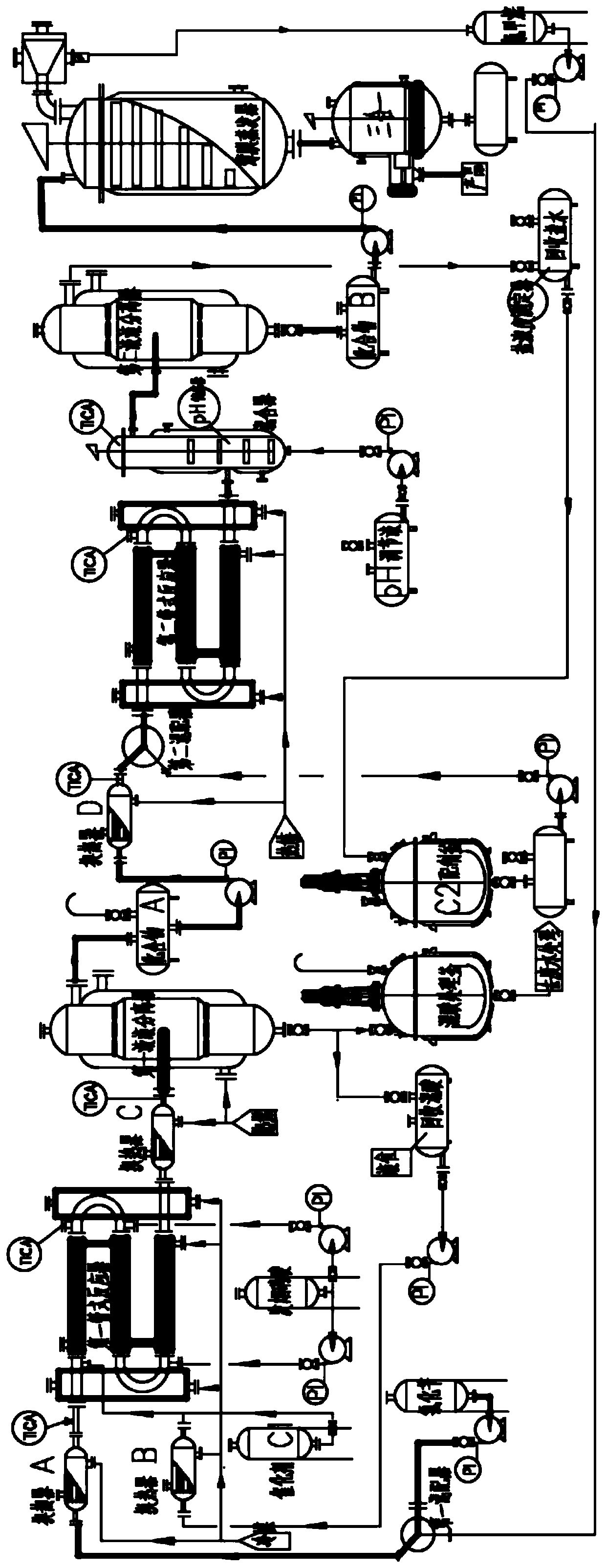
Method for continuously producing p-nitrobenzyl alcohol in tubular reactor
A tubular reactor, technology of p-nitrobenzyl alcohol, applied in chemical instruments and methods, series/parallel reaction, preparation of nitro compounds, etc., can solve the problems of long reaction time, low heat and mass transfer efficiency, etc. Improved safety factor, simplified post-processing process, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Using benzyl chloride as the benzyl halide, dichloromethane as the alkyl halide, sulfur trioxide as the acidic dehydrating agent, and sodium hydroxide as the base, in figure 1 The continuous production test in the production equipment shown is 3h, and the process conditions adopted are as follows:
[0078] a> the feed flow rate of benzyl chloride is set at 1.77Kg / min;
[0079] b> the weight ratio of each raw material is set as, benzyl chloride: methylene chloride: mixed acid: sulfur trioxide: concentrated nitric acid: water (or reclaimed water): sodium hydroxide=100:670:350:32:55:250 :38;
[0080] c>The reaction temperature is set as: nitrification reaction (first tubular reactor) temperature 0~5℃, hydrolysis reaction (second tubular reactor) temperature 35~40℃;
[0081] d> Crystallization and filtration temperature: the internal temperature of the three-in-one is -13~-15°C;
[0082] e> Drying temperature, vacuum degree and time: the internal temperature of the three...
Embodiment 2
[0085] With embodiment 1, benzyl bromide is used as benzyl halide, ethylene dichloride as alkyl halide, sulfur trioxide as acidic dehydrating agent, without alkaline / neutral / acidic aqueous solution, in figure 1 The continuous production test in the production equipment shown is 3h, and the process conditions adopted are as follows:
[0086] a> the feed flow rate of benzyl chloride is set at 2.40Kg / min;
[0087] b> the weight ratio of each raw material is set as, benzyl bromide: methylene chloride: mixed acid: sulfur trioxide: concentrated nitric acid: water (or recycled water) = 100: 600: 250: 24: 42: 300;
[0088] c>The reaction temperature is set as: nitration reaction (first tubular reactor) temperature 10-15°C, hydrolysis reaction (second tubular reactor) temperature 70-75°C;
[0089] d> Crystallization and filtration temperature: the internal temperature of the three-in-one is set to -13~-15°C;
[0090] e> Drying temperature, vacuum degree and time: the internal tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com